No products in the cart.
Paracetamol EP Impurity L

Product Description
CAT No.
ALN-P007015
CAS No.
2514961-29-4
Mol. F.
C16H16N2O4
Mol. Wt.
300.3
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
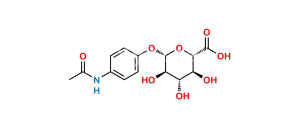
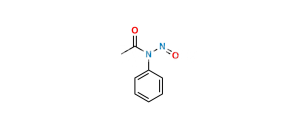
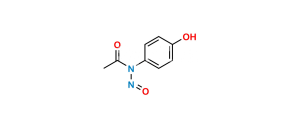
Chemical Name : N-[4-(4-acetamido-2-hydroxyphenoxy)phenyl]acetamide
Smiles : CC(NC1=CC=C(OC2=CC=C(NC(C)=O)C=C2O)C=C1)=O
Inchi : InChI=1S/C21H28O6/c1-19-6-5-12(23)7-11(19)3-4-13-14-8-16(25)21(27,17(26)10-22)20(14,2)9-15(24)18(13)19/h5-7,13-16,18,22,24-25,27H,3-4,8-10H2,1-2H3/t13-,14-,15-,16-,18+,19-,20-,21-/m0/s1
Technical Data
Reference
Thermodynamic properties of paracetamol impurities 4-nitrophenol and 4u2032-chloroacetanilide and the impact of such impurities on the crystallisation of paracetamol from solution
Renu00e9 R.E.Steendam, LeilaKeshavarz, Briande Souza, Patrick J.FrawleynThe Journal of Chemical Thermodynamics Volume 133, June 2019, Pages 85-92
Impact of Paracetamol Impurities on Face Properties: Investigating the Surface of Single Crystals Using TOF-SIMS
Sara Ottoboni, Michael Chrubasik, Layla Mir Bruce, Thai Thu Hien Nguyen, Murray Robertson, Blair Johnston,Iain D. H. Oswald, Alastair Florence, and Chris Price – Cryst. Growth Des. 2018, 18, 5, 2750–2758
Separation and determination of impurities in paracetamol, codeine and pitophenone in the presence of fenpiverinium in combined suppository dosage form
Ji?í Vojta, Pavel Hanzlík, Aleš Jedli?ka, Pavel Coufal – J Pharm Biomed Anal. 2015 Jan;102:85-92.
RFQ