No products in the cart.
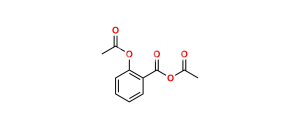
Acetylsalicylic Acid EP Impurity E (Aspirin Impurity E)

Product Description
CAT No.
ALN-A046005
CAS No.
552-94-3
Mol. F.
C14H10O5
Mol. Wt.
258.2
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
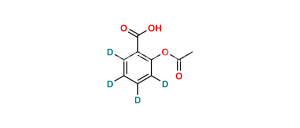
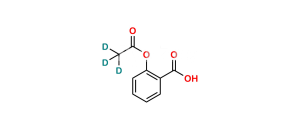
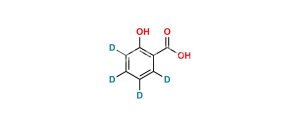
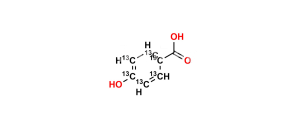
Chemical Name : Salsalate; Aspirin Impurity E, 2-Hydroxybenzoic Acid 2-Carboxyphenyl Ester; Salicylic Acid Salicylate; 2-Hydroxybenzoic Acid 2-Carboxyphenyl Ester; Diacesal; Diplosal; Disalcid; Disalgesic; Disalicylic Acid; Disalyl; Mono-Gesic; NSC 49171; Nobacid; Salflex; Salical; Salicyl Salicylate; Salicyloxysalicylic Acid; Salicyloylsalicylic Acid; Salicylsalicylic Acid; Salina; Salsalate; Salysal; Sasapirin; Sasapyrine; Sasapyrinum; o-Salicylsalicylic Acid;
Smiles : O=C(OC1=CC=CC=C1C(O)=O)C2=CC=CC=C2O
Inchi : InChI=1S/C16H12O6/c1-10(17)21-14-9-5-3-7-12(14)16(20)22-13-8-4-2-6-11(13)15(18)19/h2-9H,1H3,(H,18,19)
Technical Data
Reference
Aspirin-A National Survey V: Determination of Aspirin and Impurities in Enteric Coated Tablets and Suppository Formulations and In Vitro Dissolution of Enteric Coated Tablets
Ross D. Kirchhoefer , Everett jefferson, and Paul e. flinnnJournal of Pharmaceutical Sciences I 1049 Vol. 71, No. 9. September 7982
Solubility of Benzoic Acid and Aspirin in Pure Solvents Using Focused Beam Reflective Measurement
Gavin Tully,† Guangyang Hou,† and Brian Glennon – J. Chem. Eng. Data 2016, 61, 1, 594–601
Isolation, Characterization of a Potential Degradation Product of Aspirin and an HPLC Method for Quantitative Estimation of Its Impurities
Subasranjan Acharya, Alex Daniel, Bharath Gyadangi, Sriramulu Ramsamy – Journal of Chromatographic Science, Volume 53, Issue 9, October 2015, Pages 1491–1497
RFQ