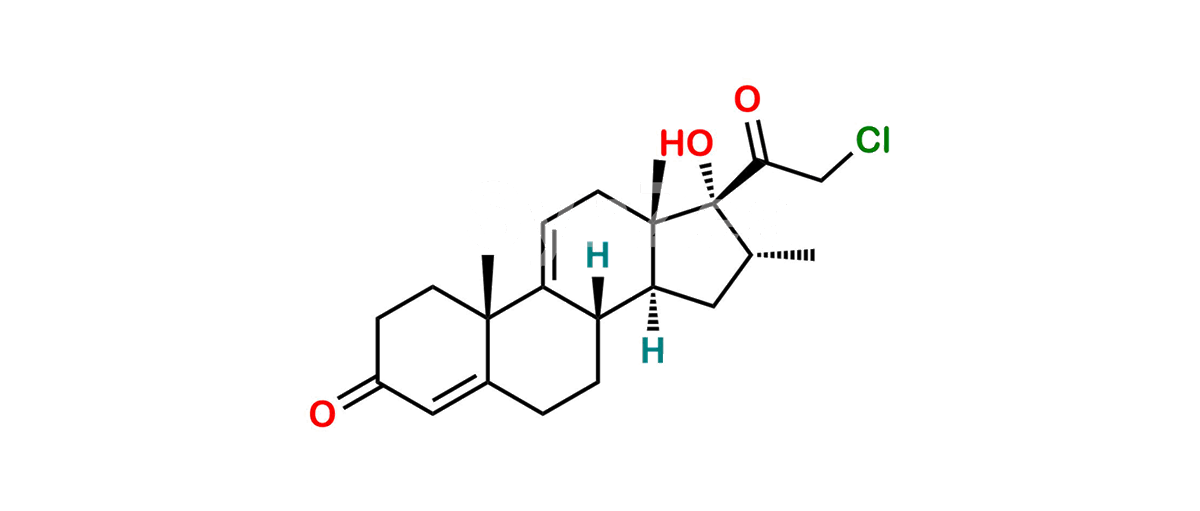
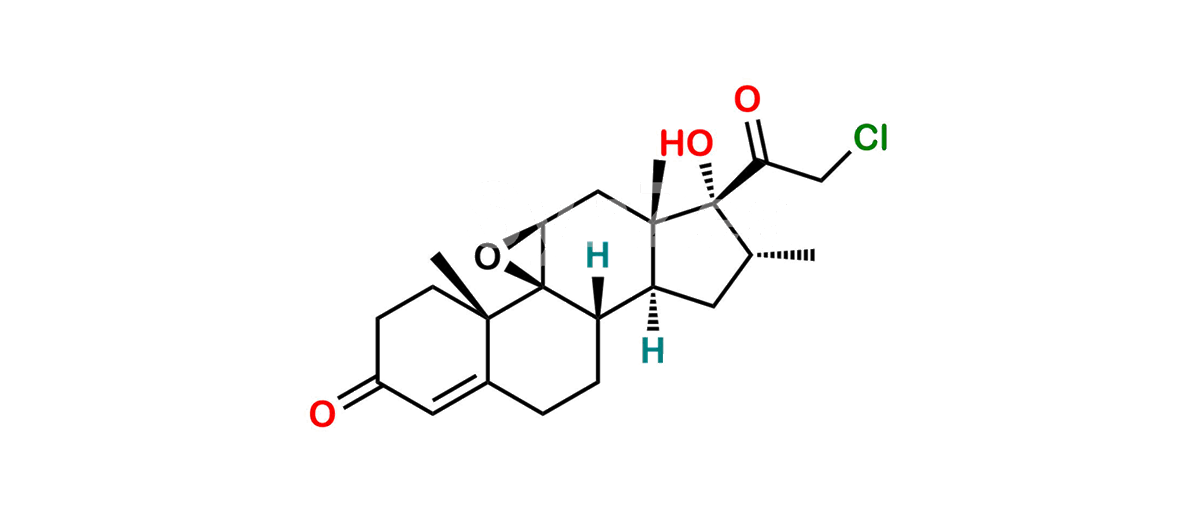
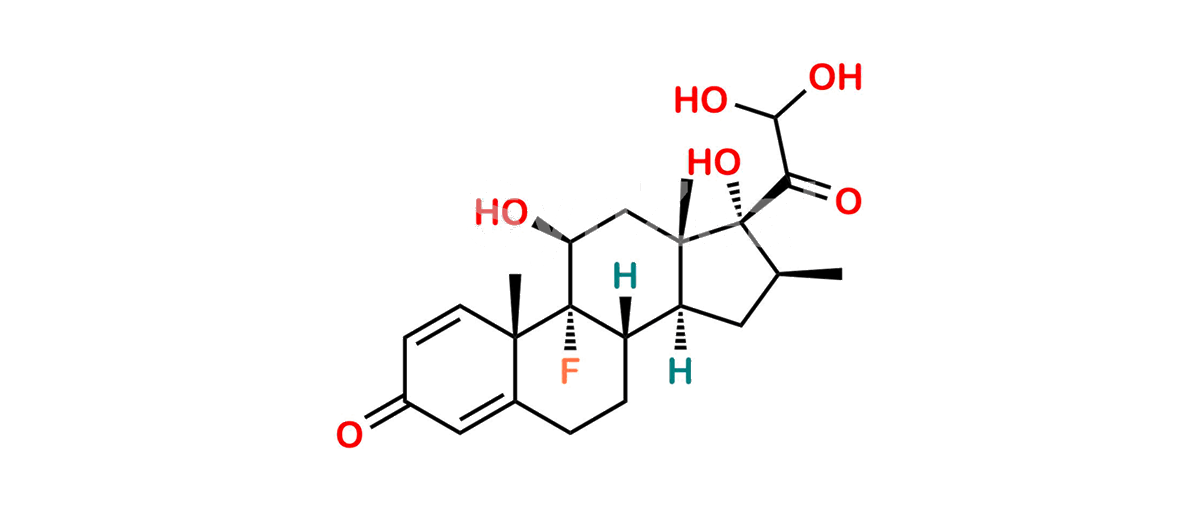
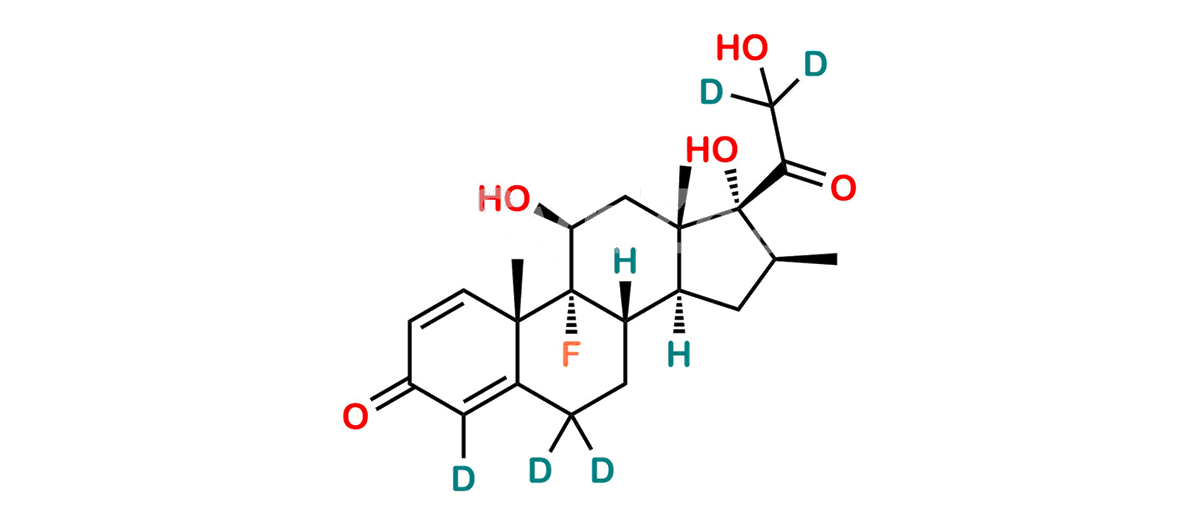
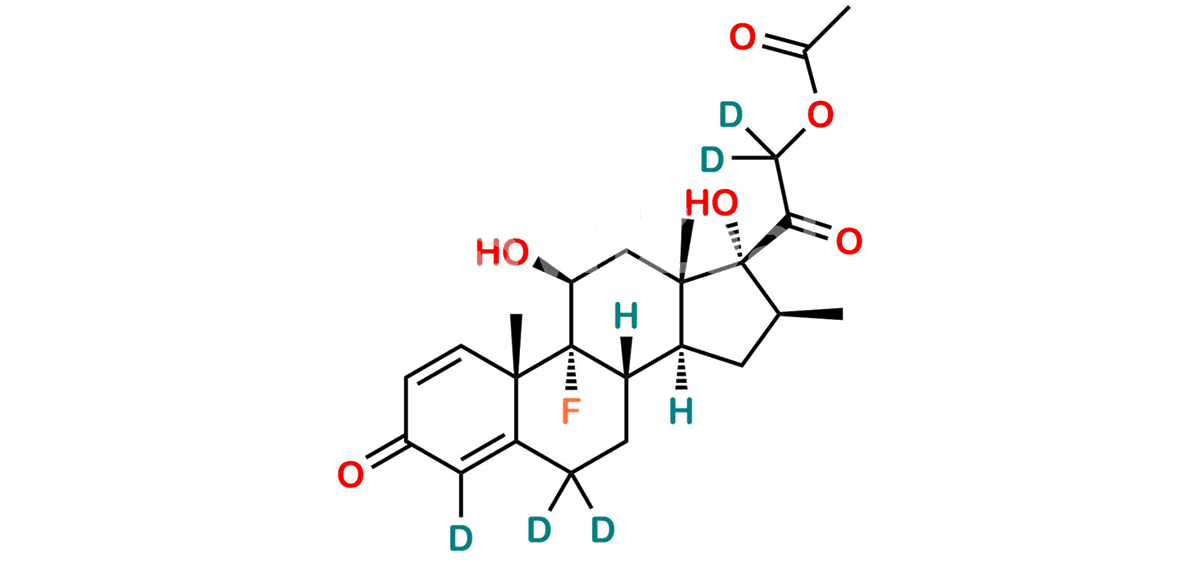
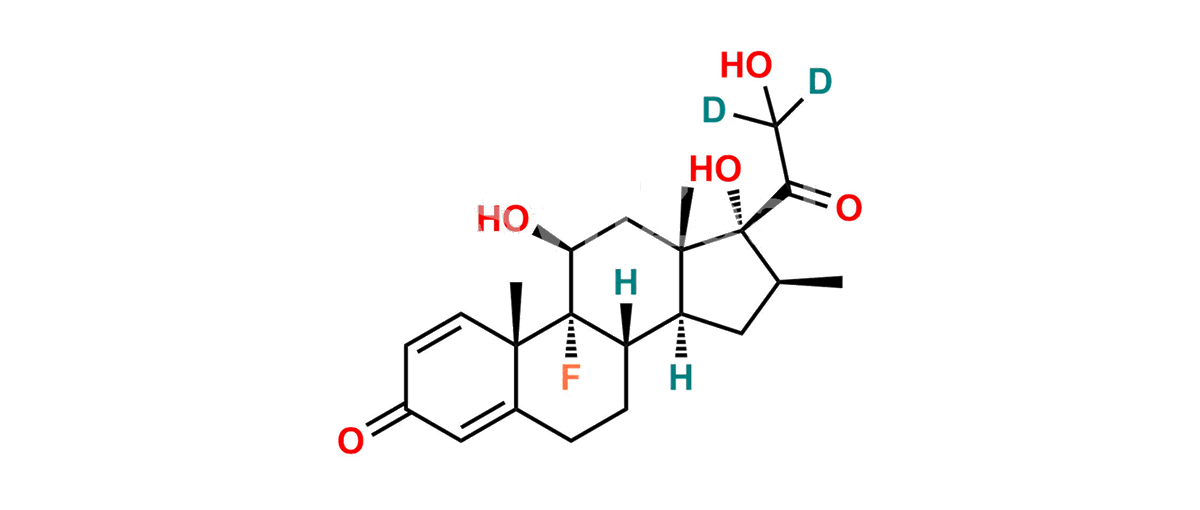
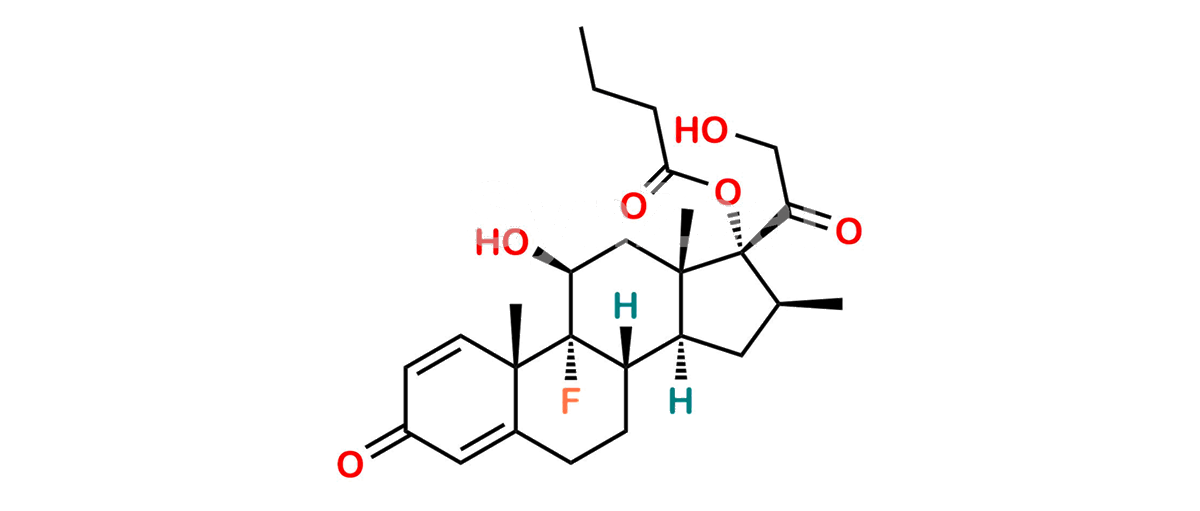
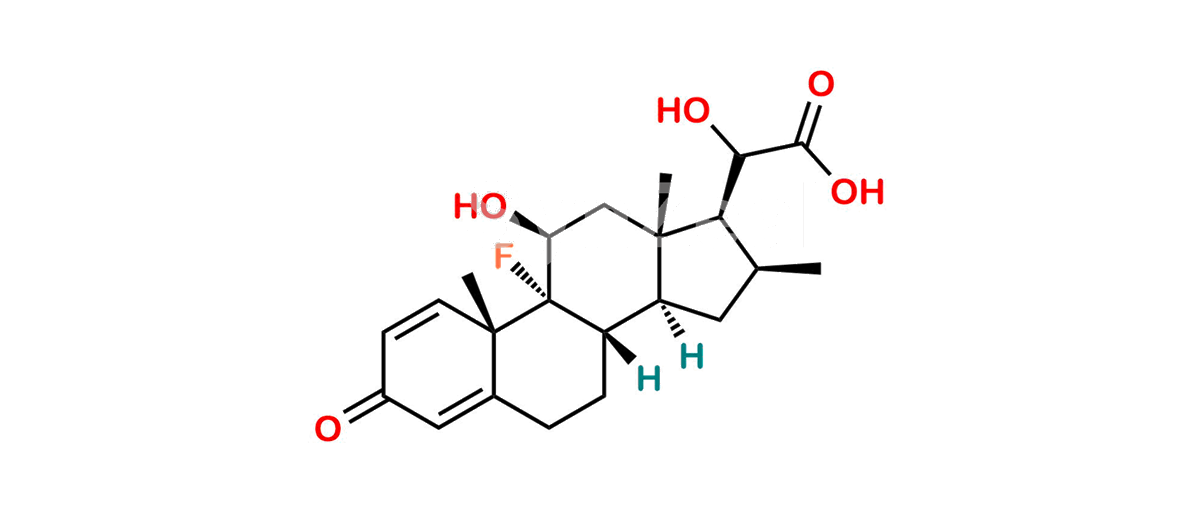
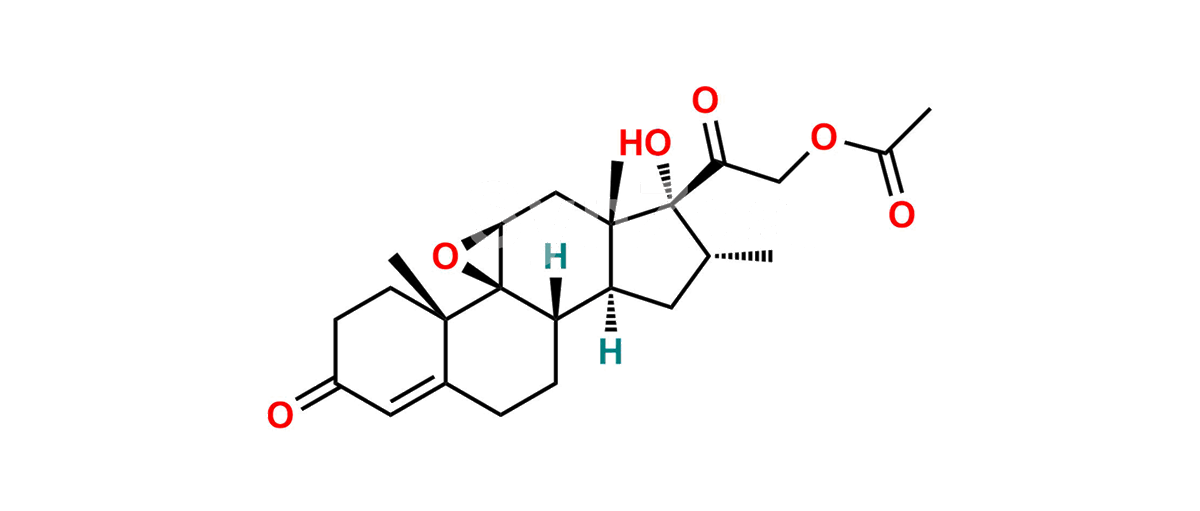
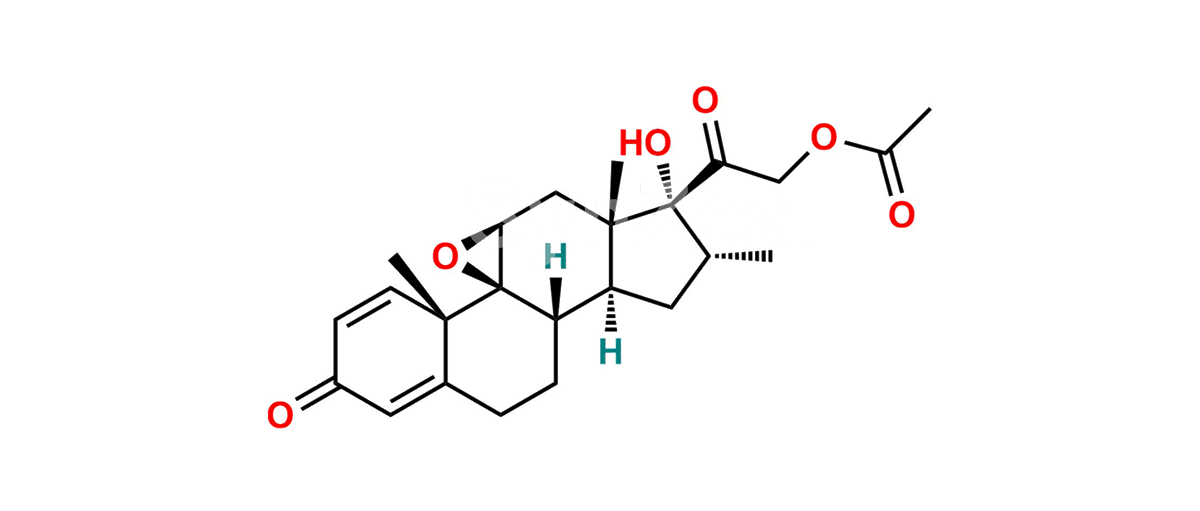
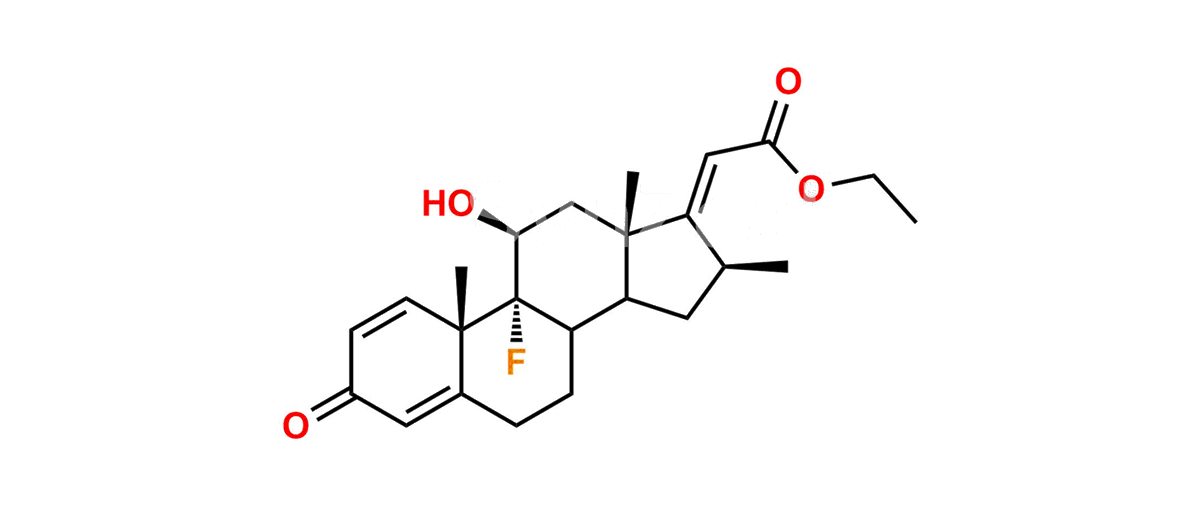
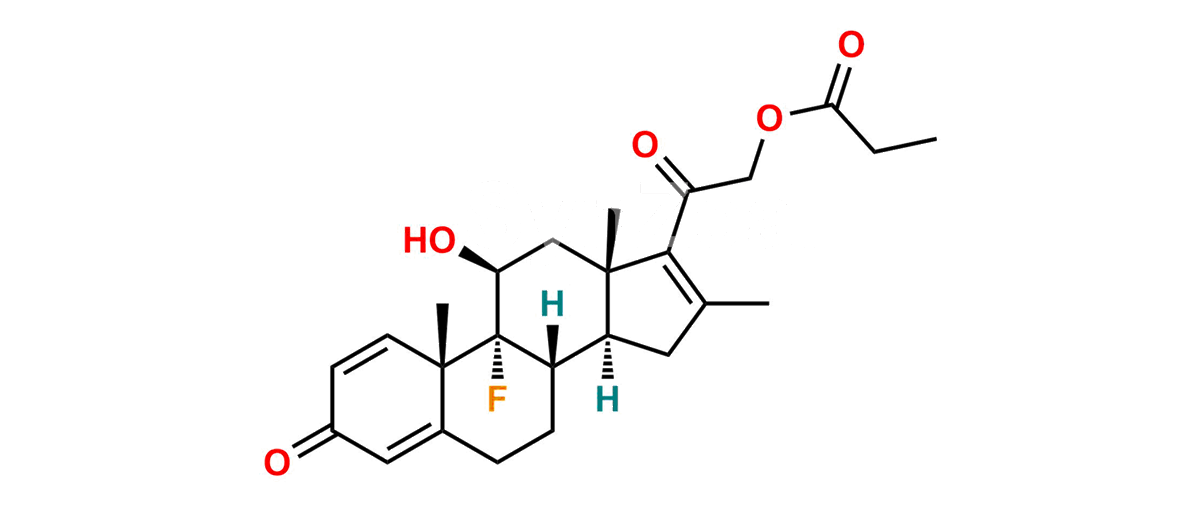
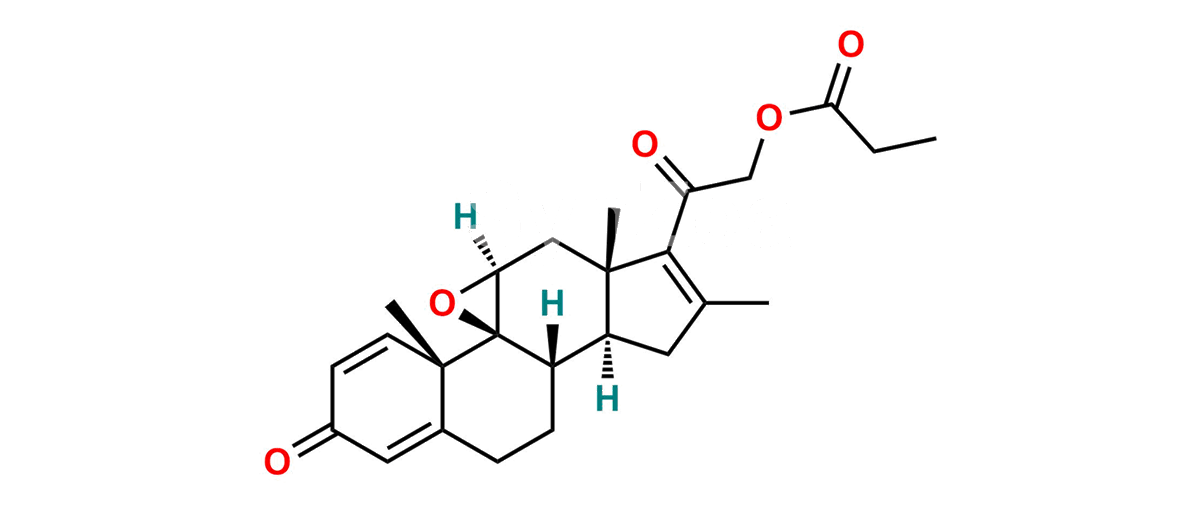
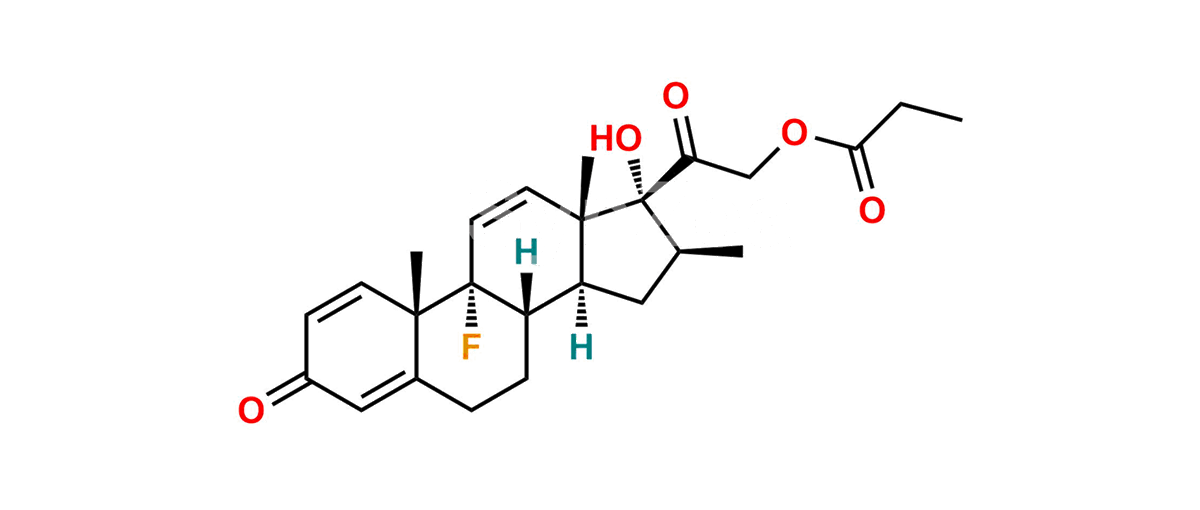
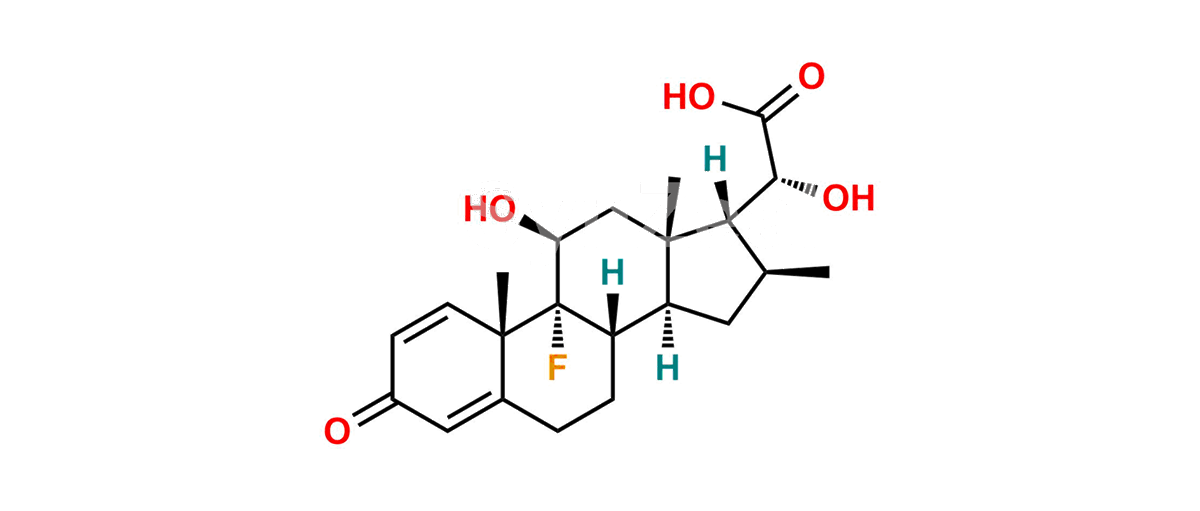
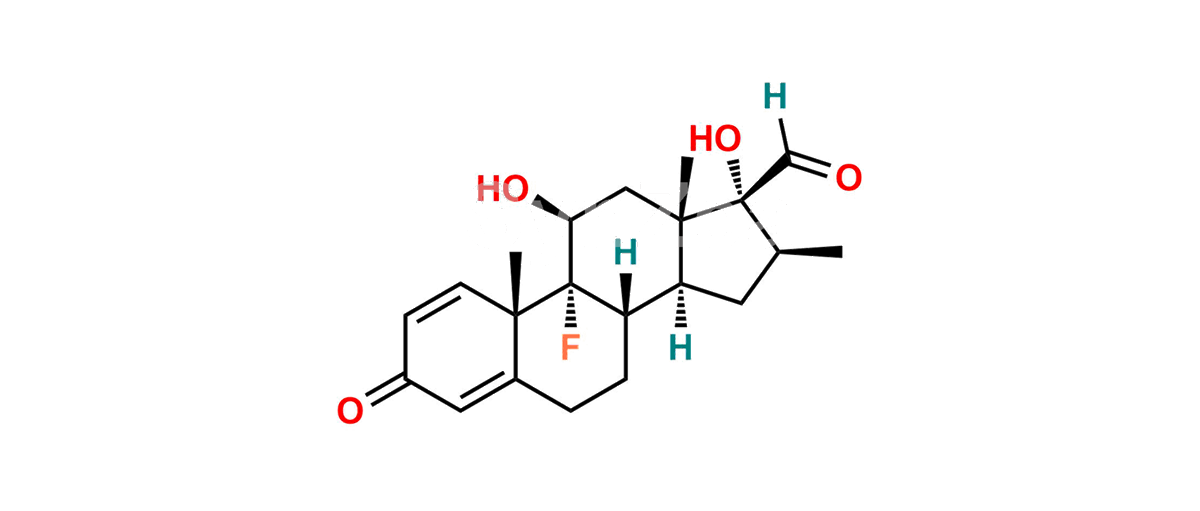
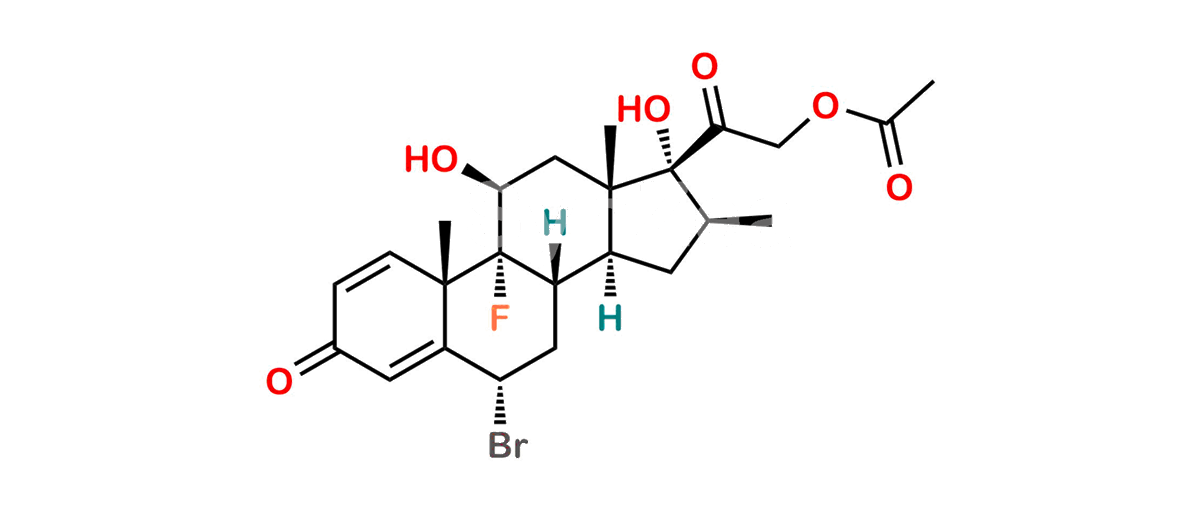
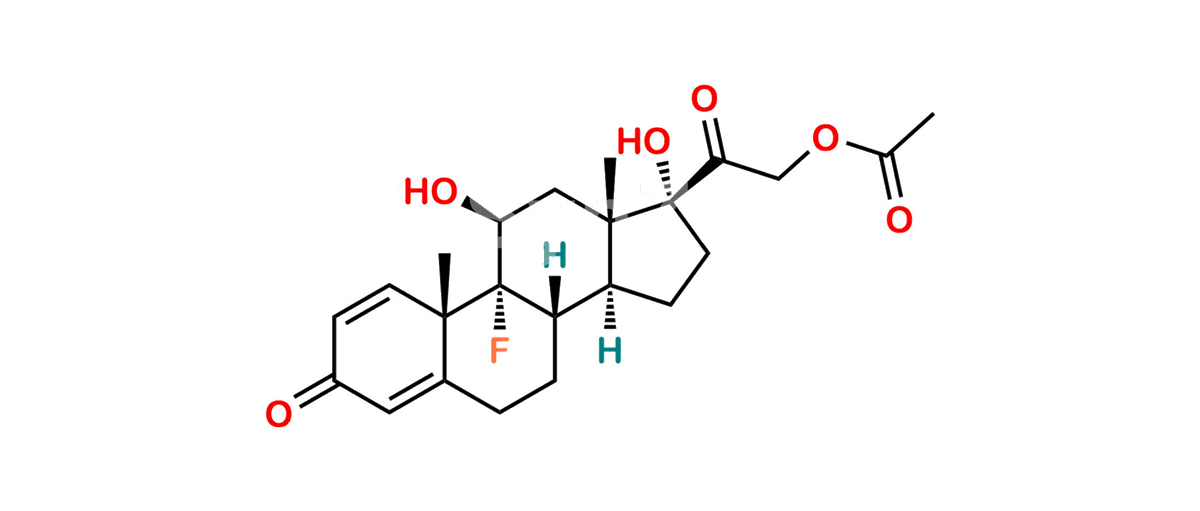
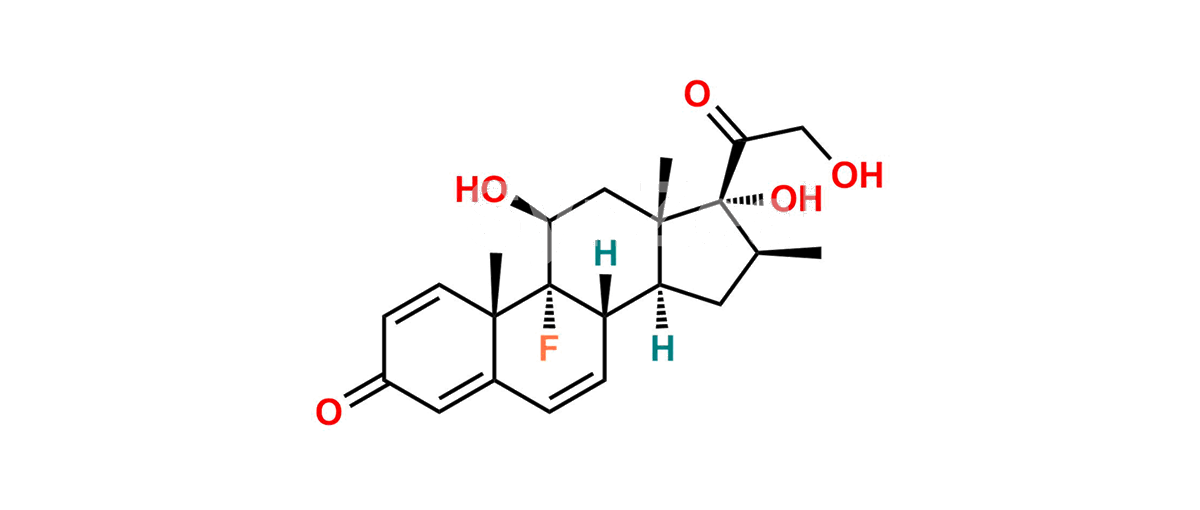
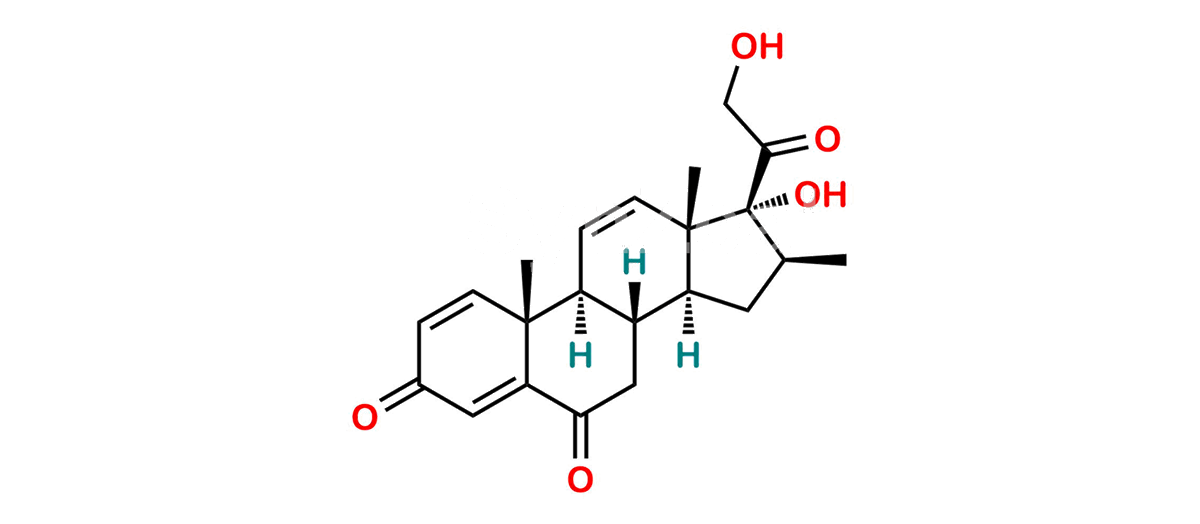
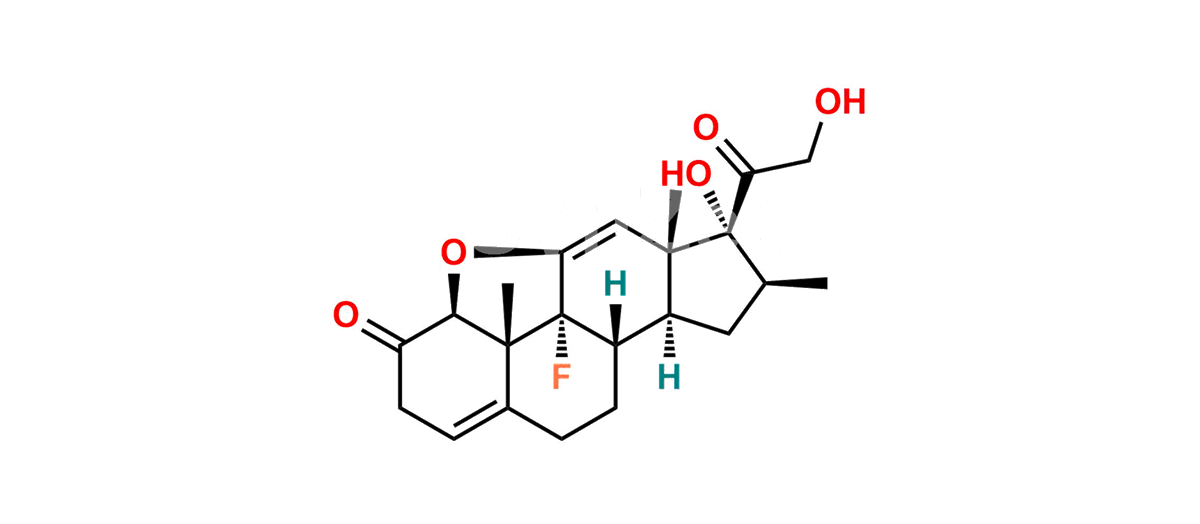
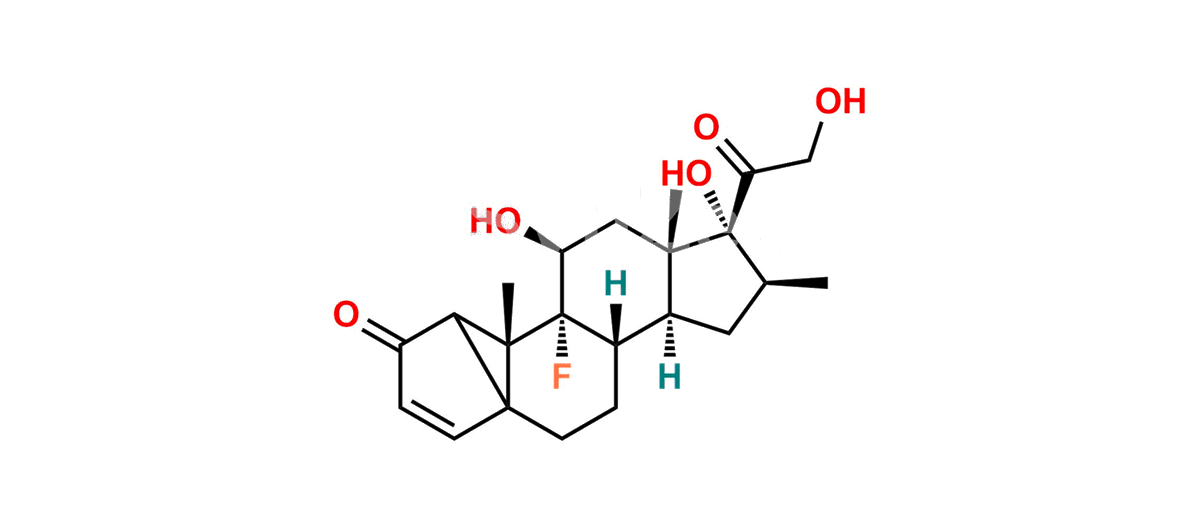
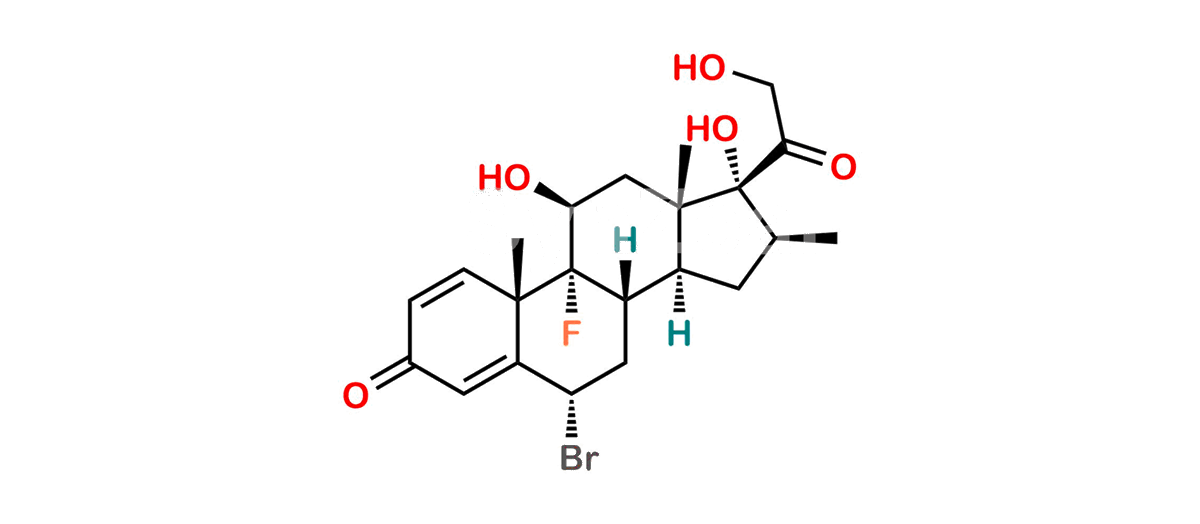
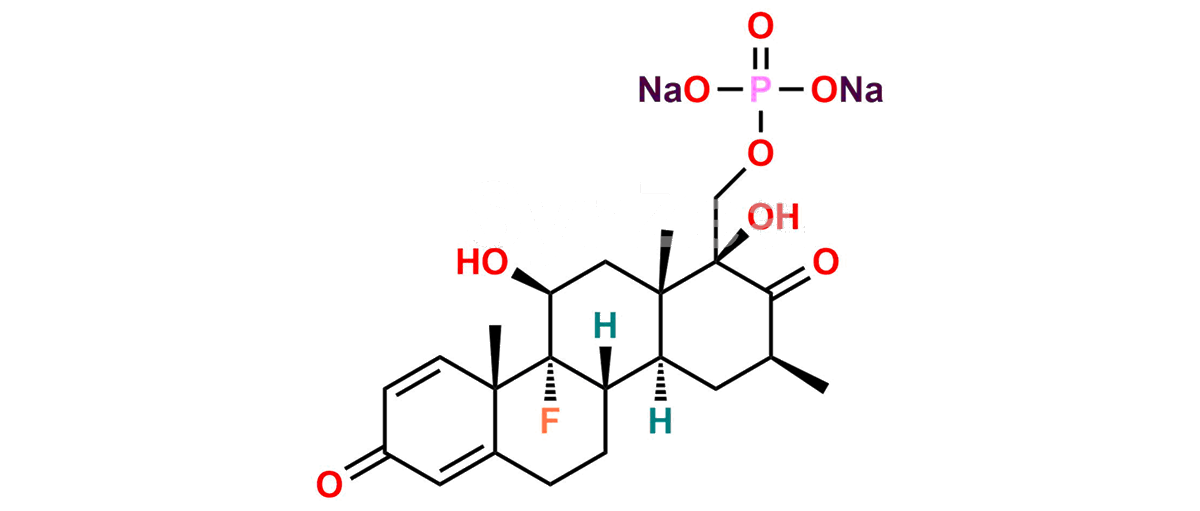
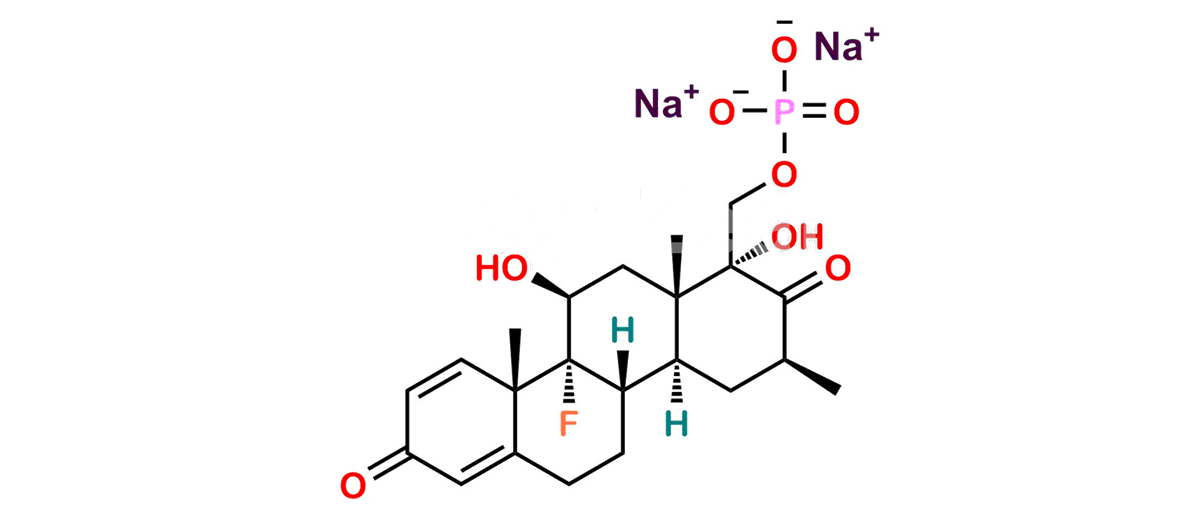
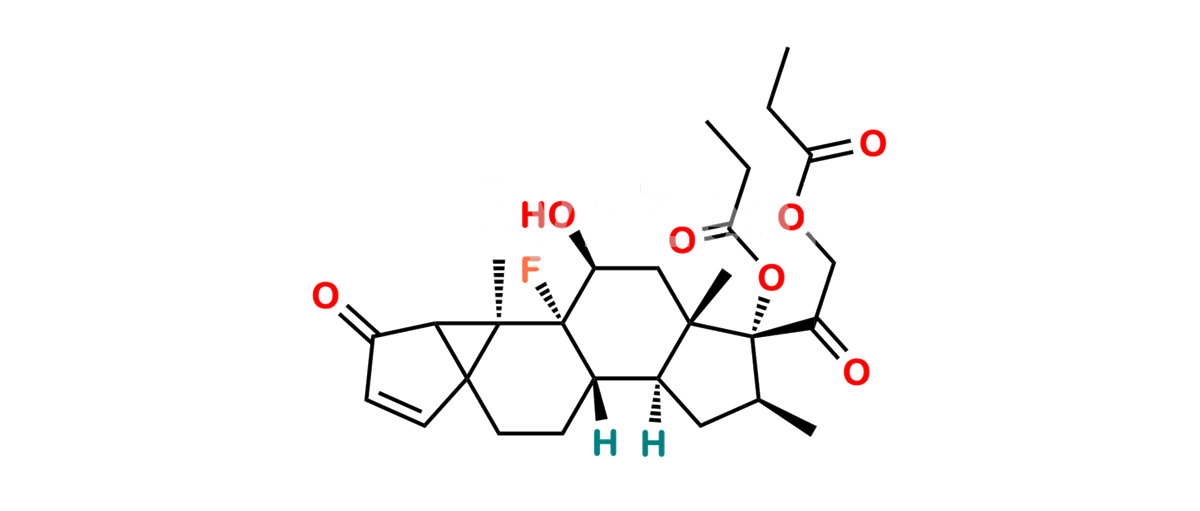
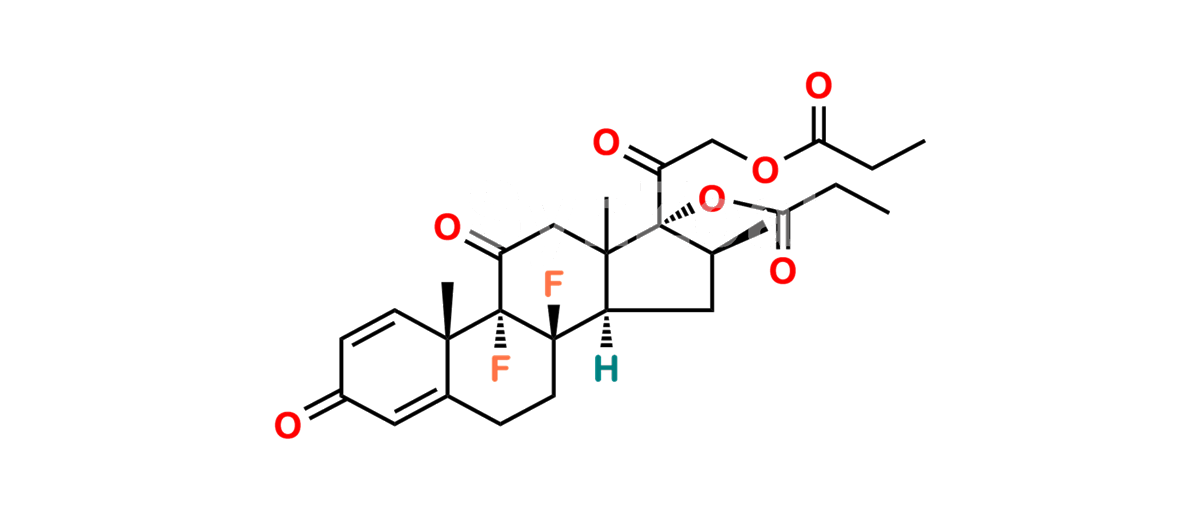
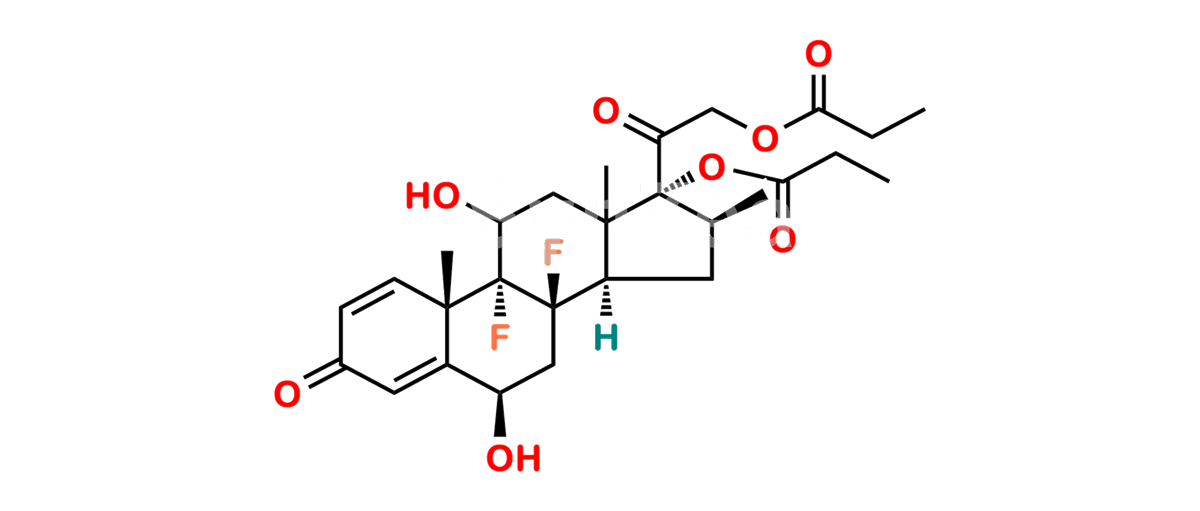
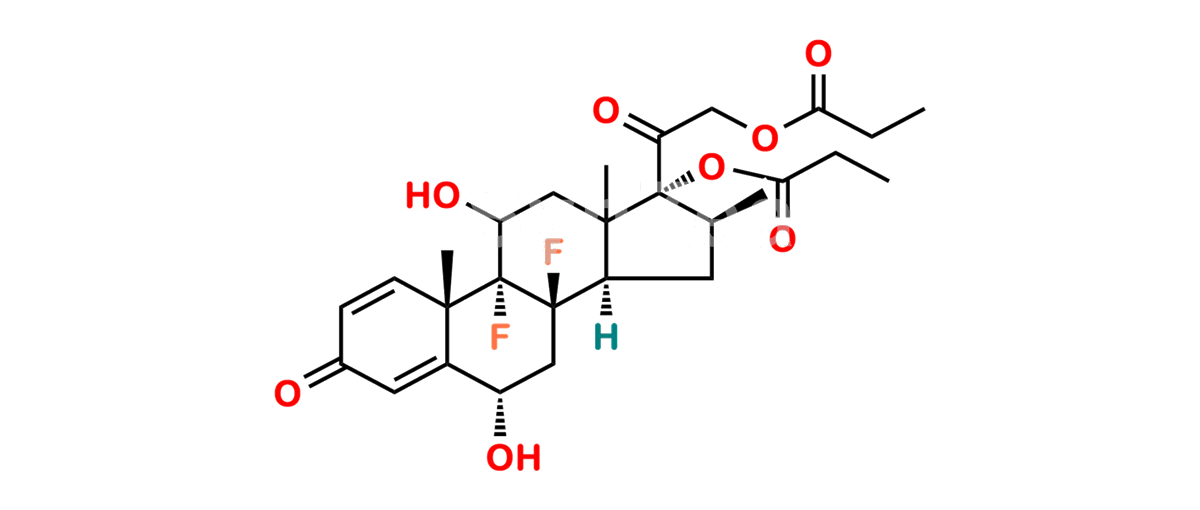
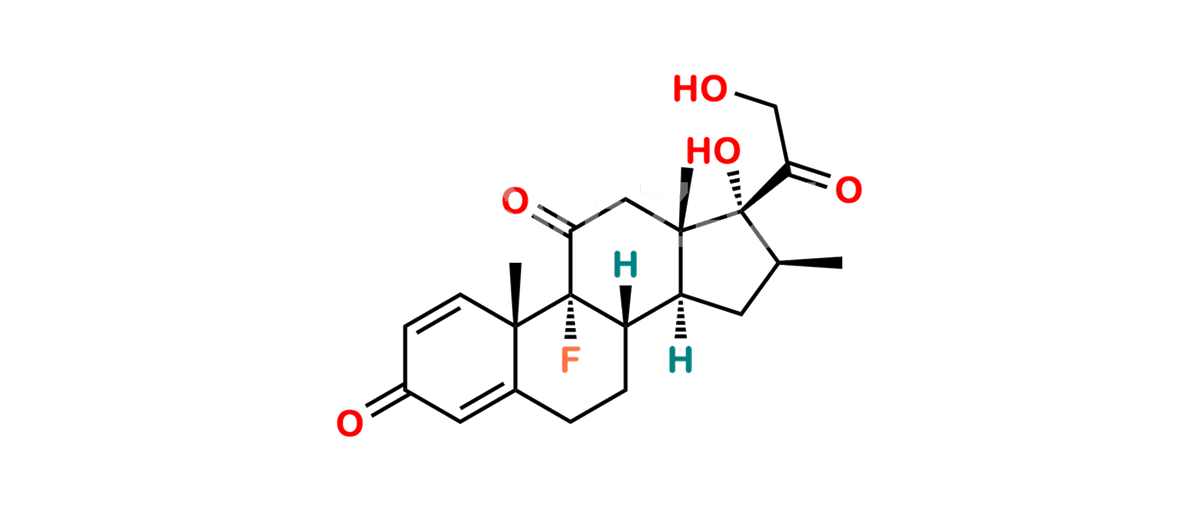
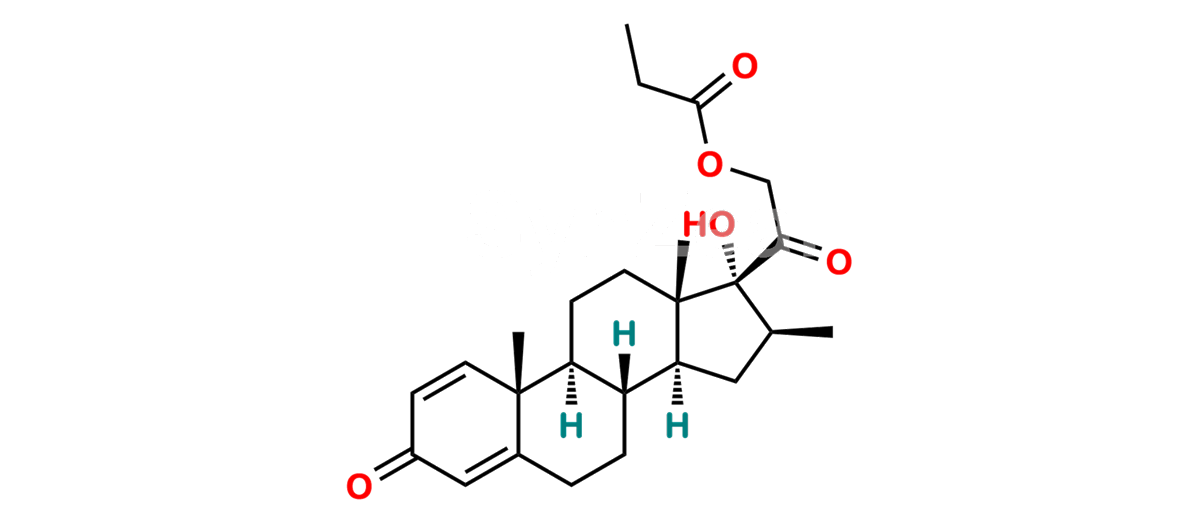
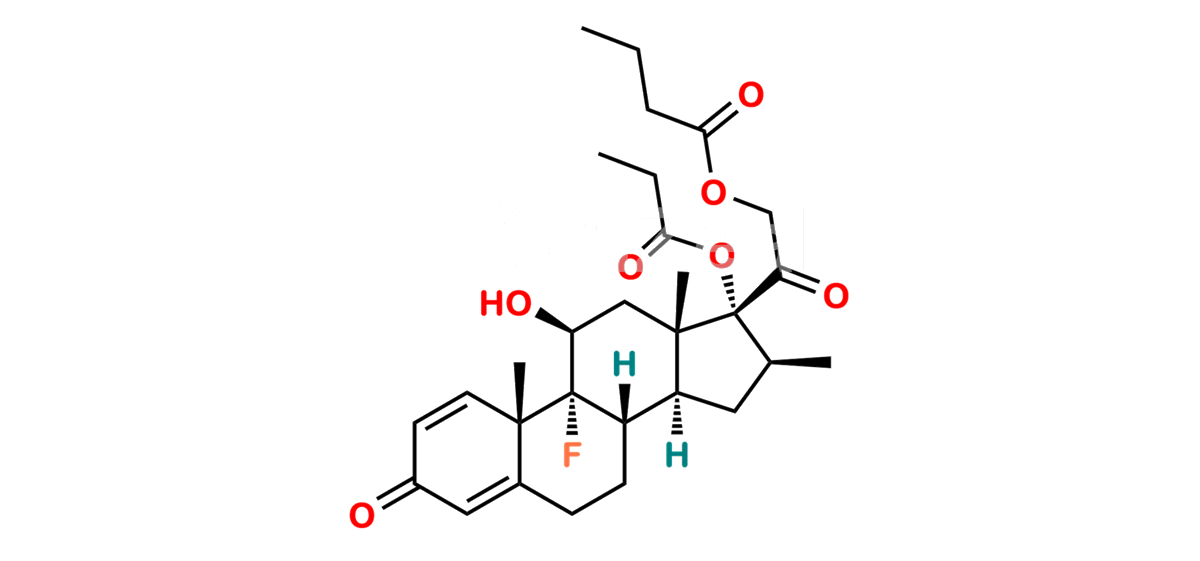
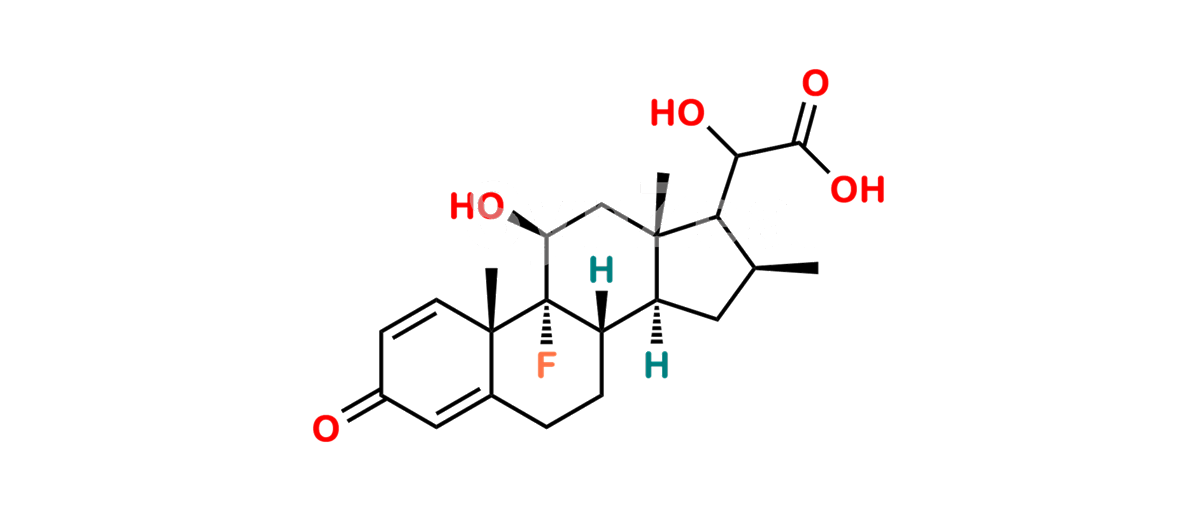
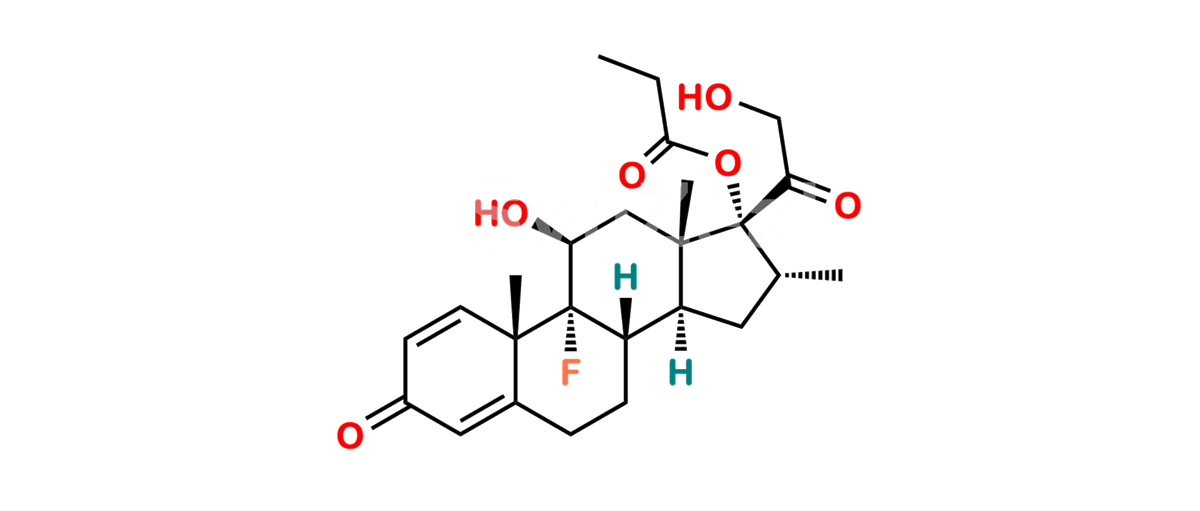
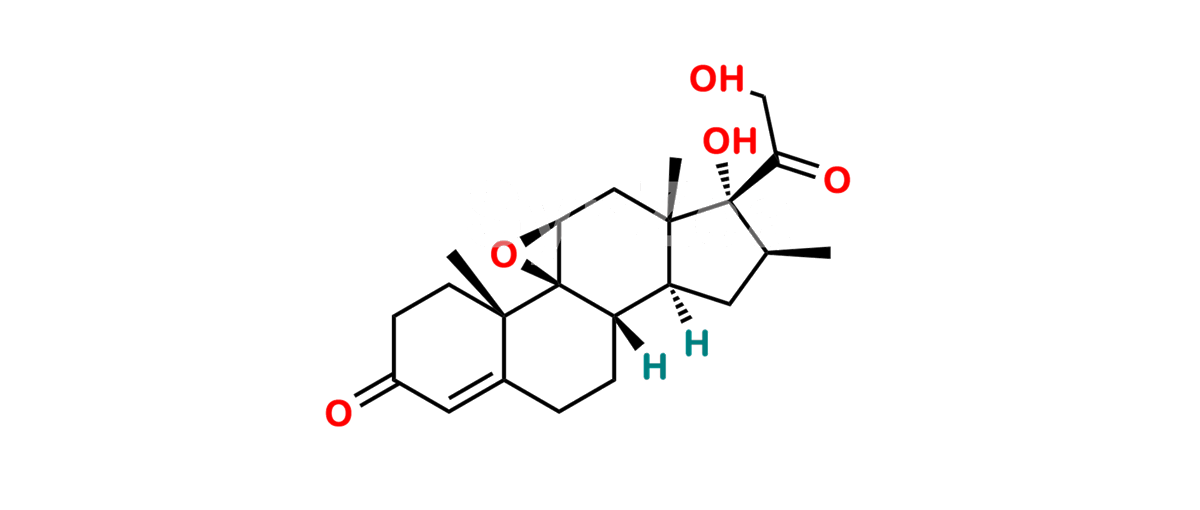
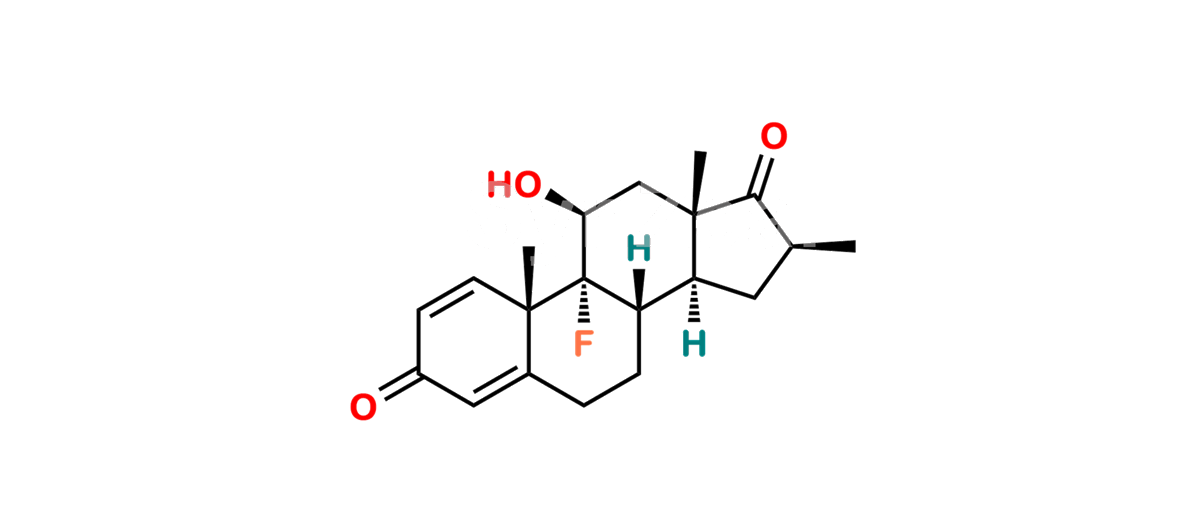
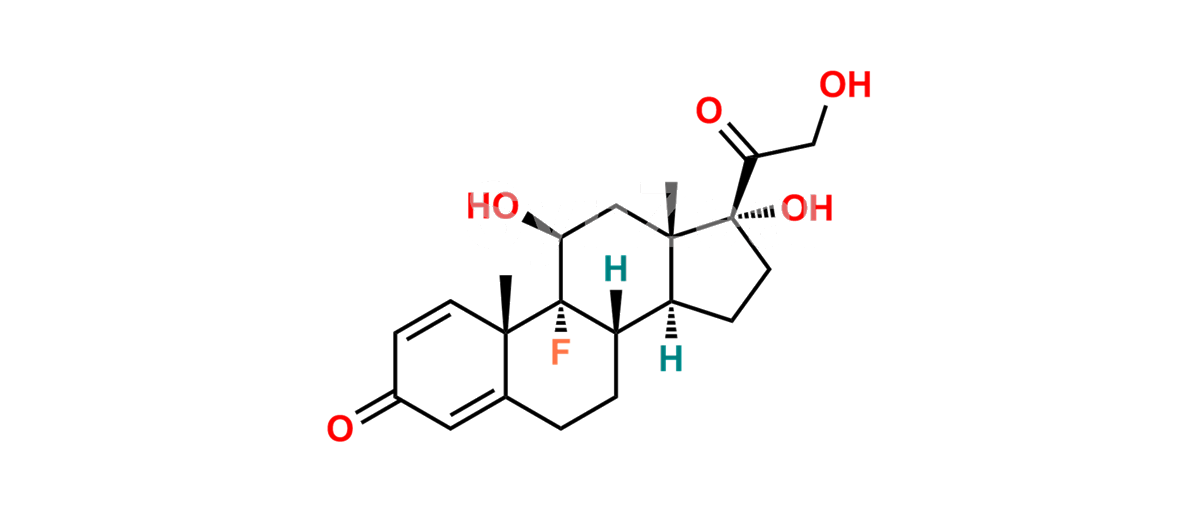
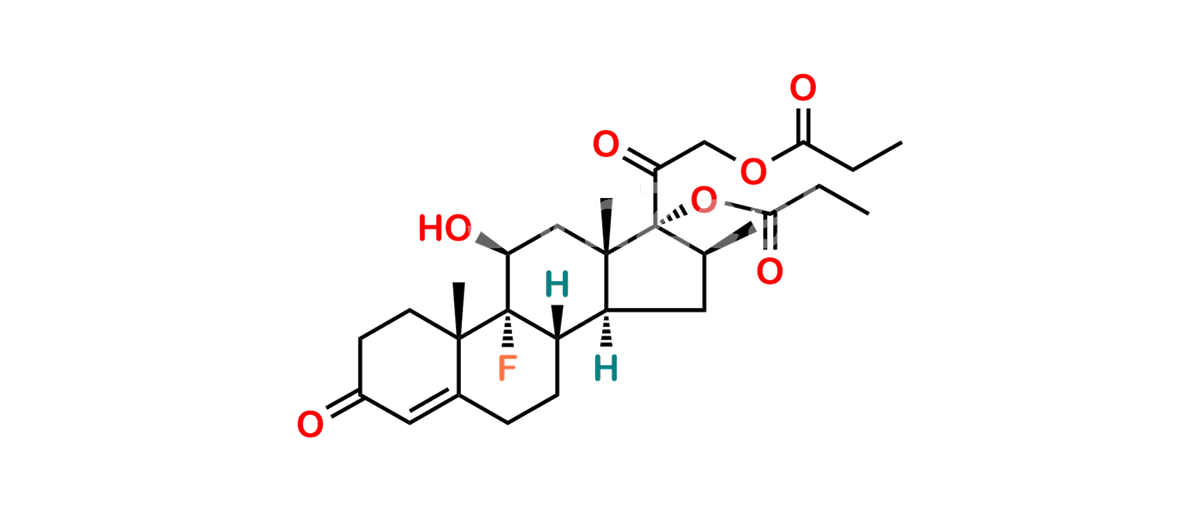
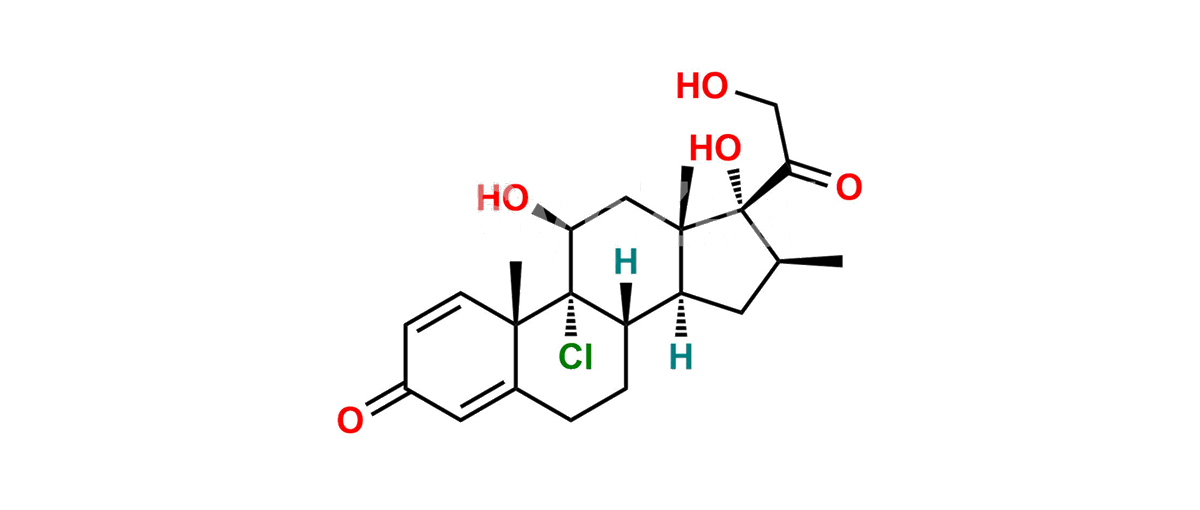
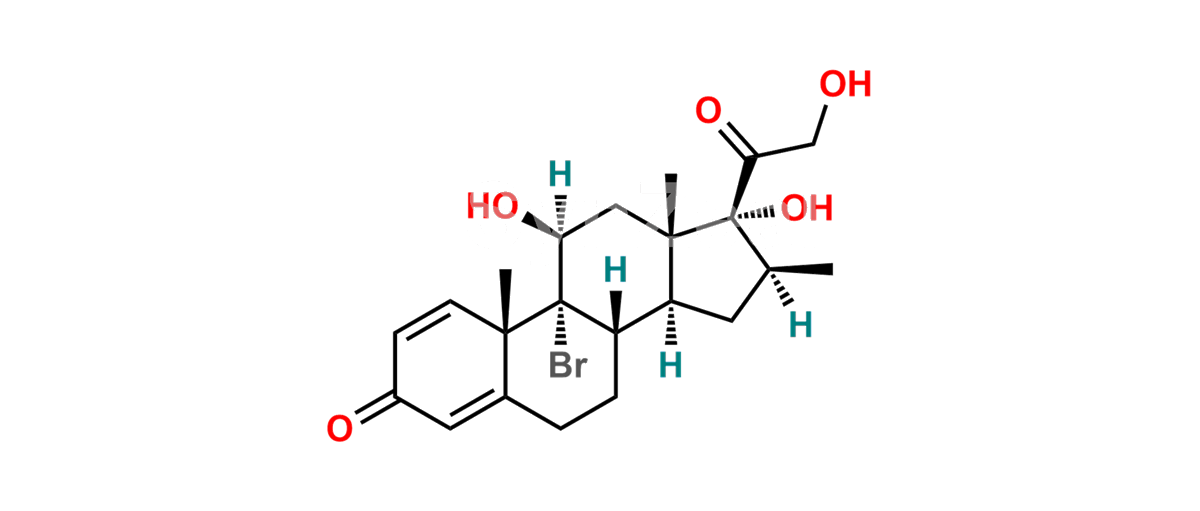
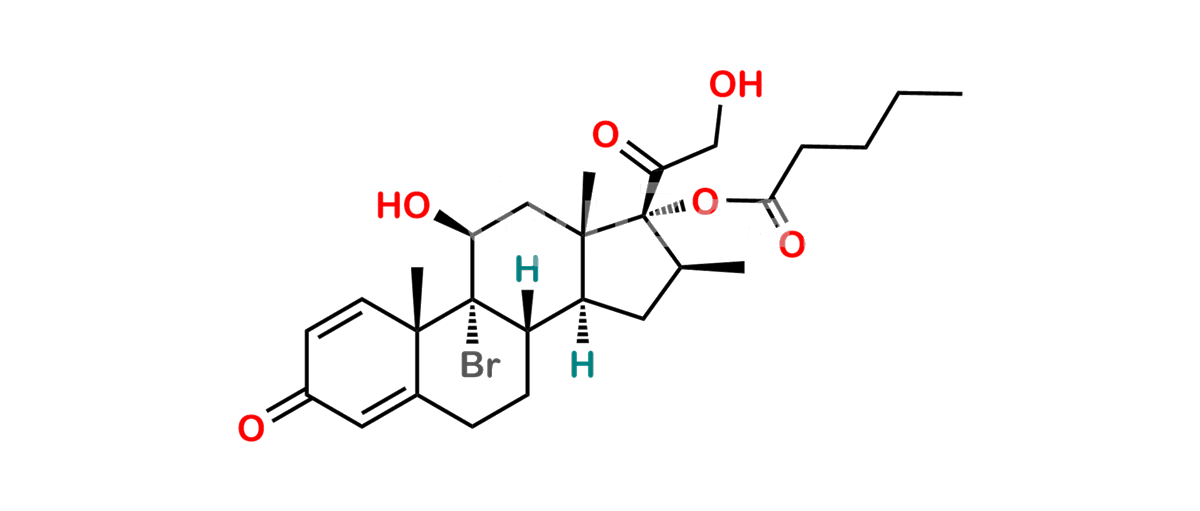
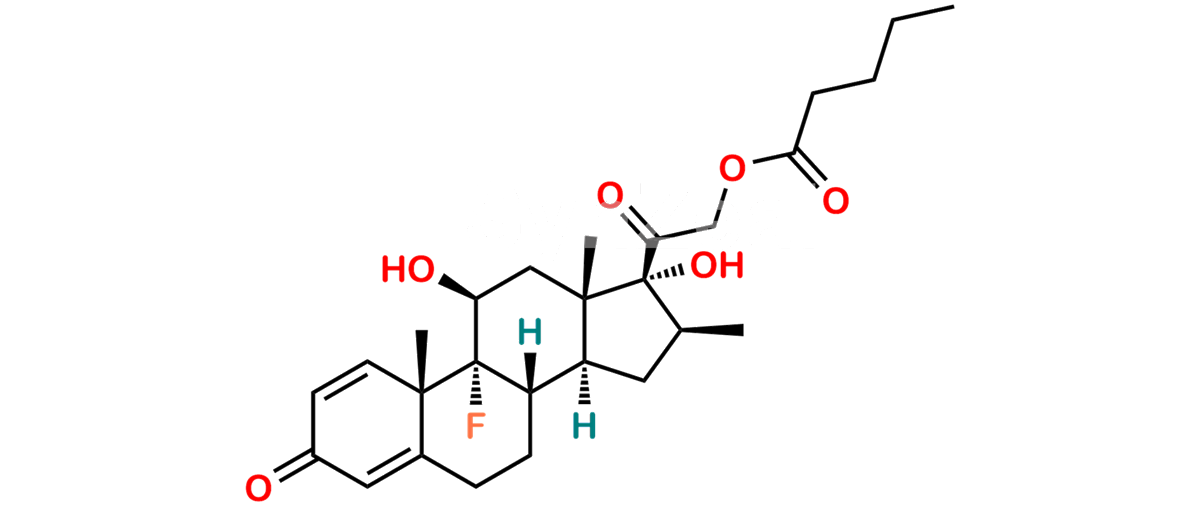
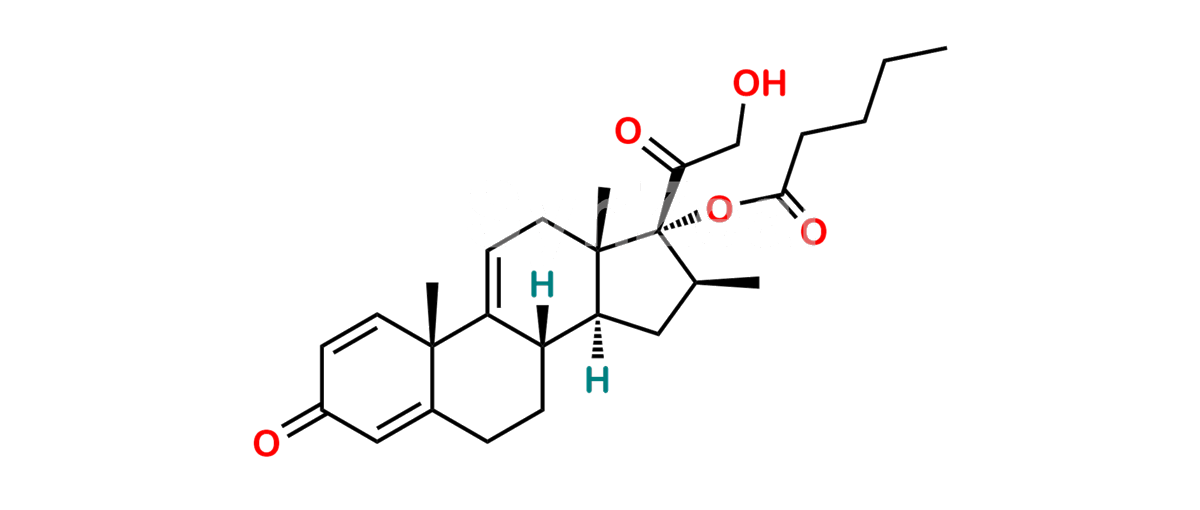
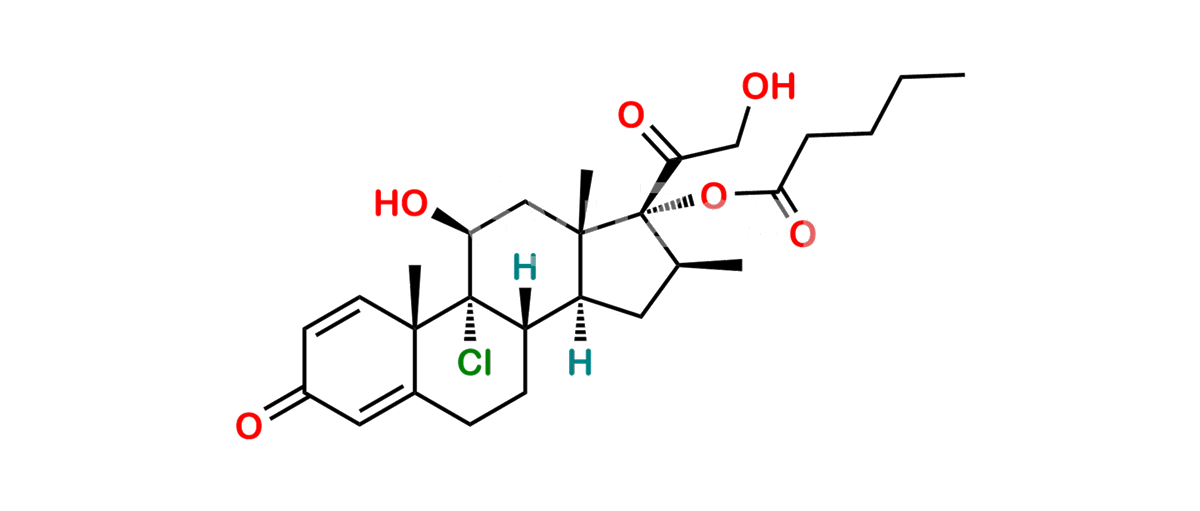
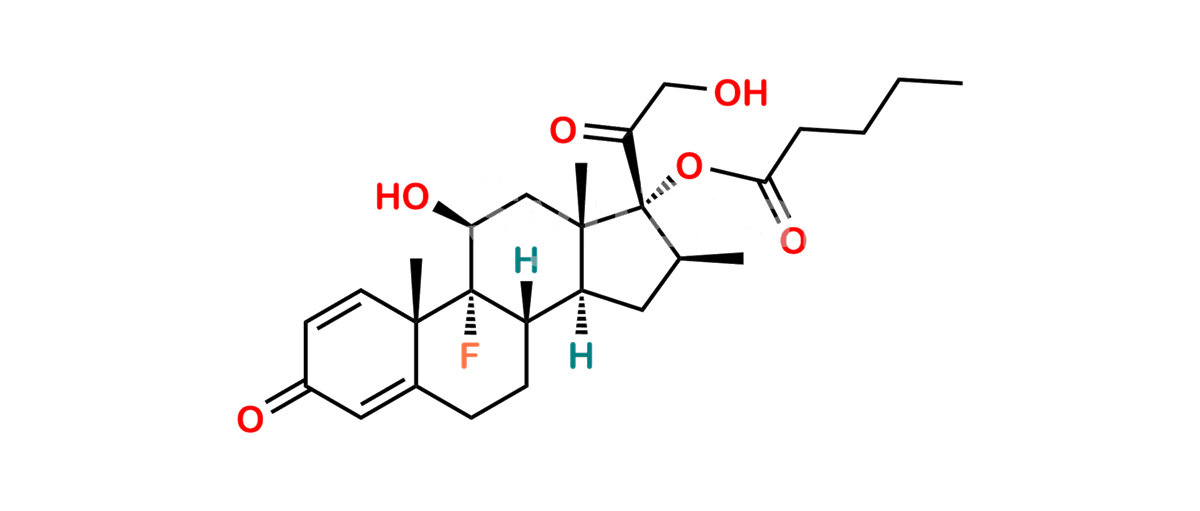
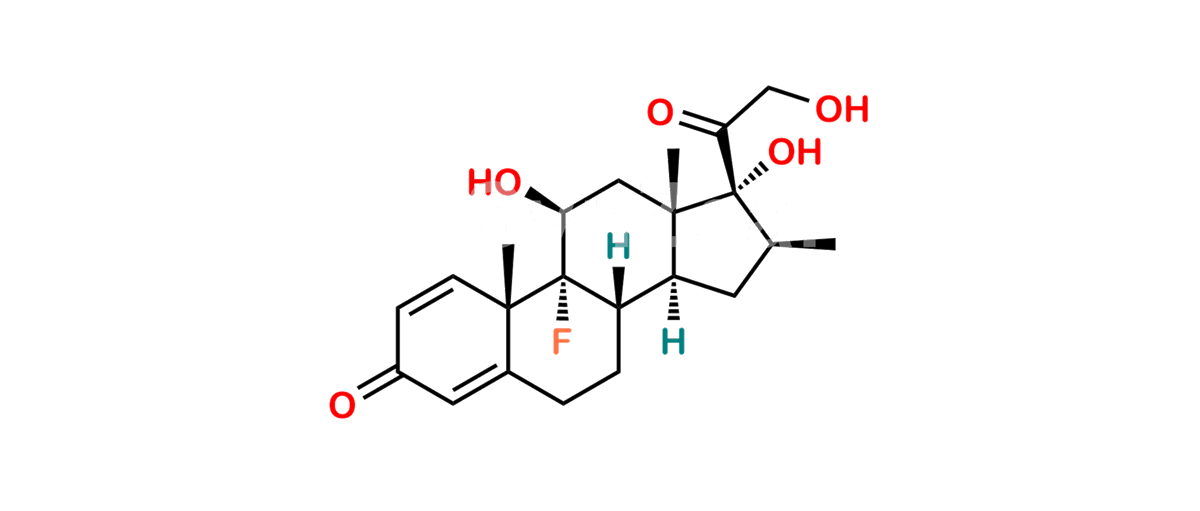
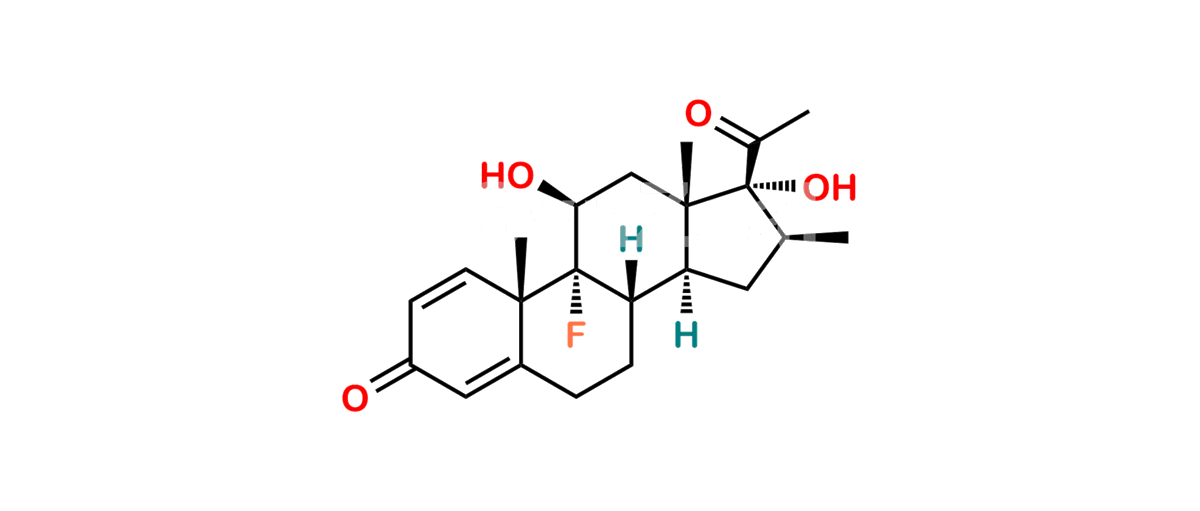
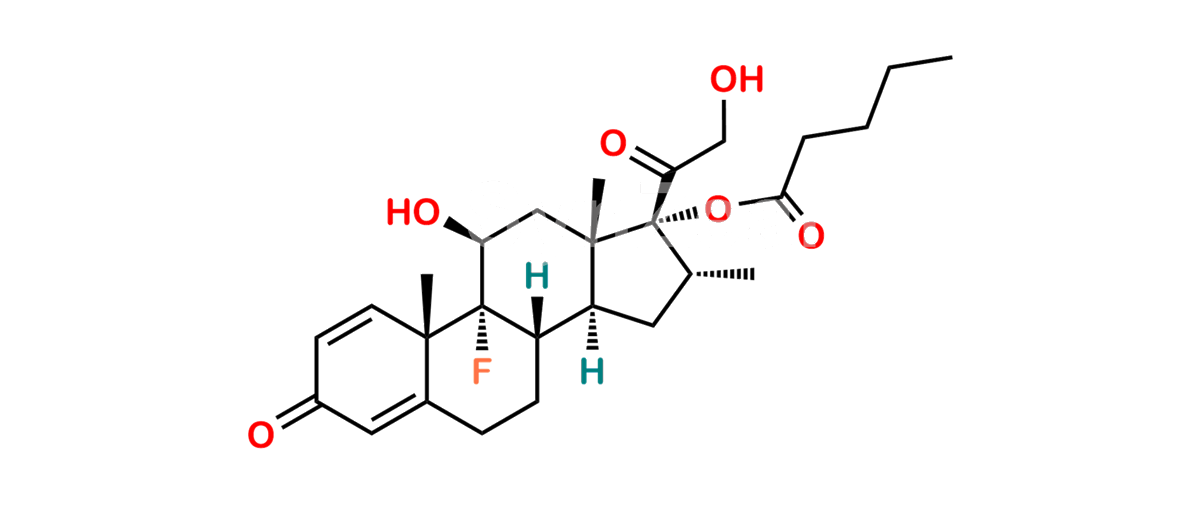
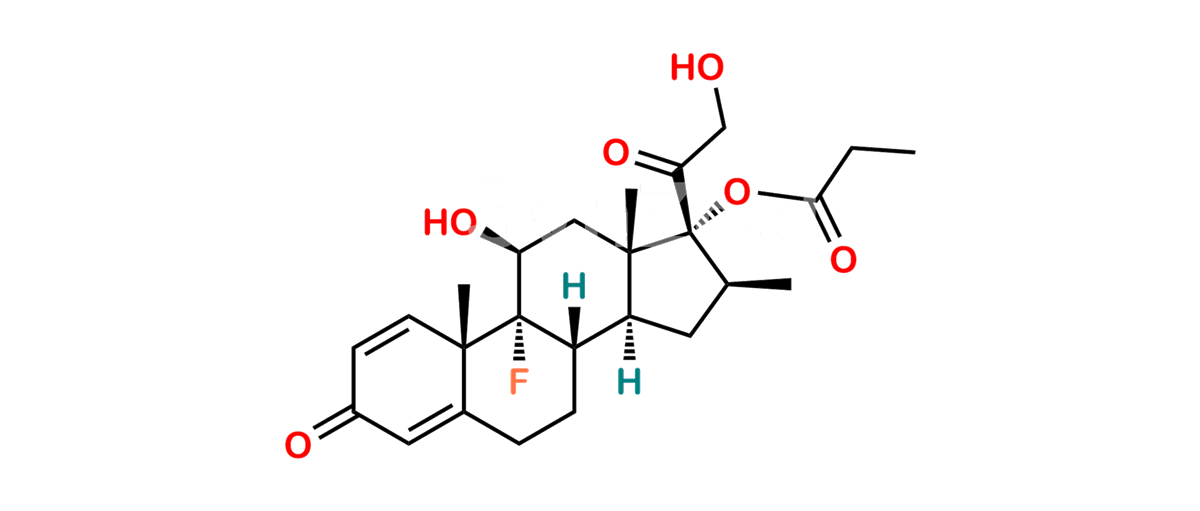
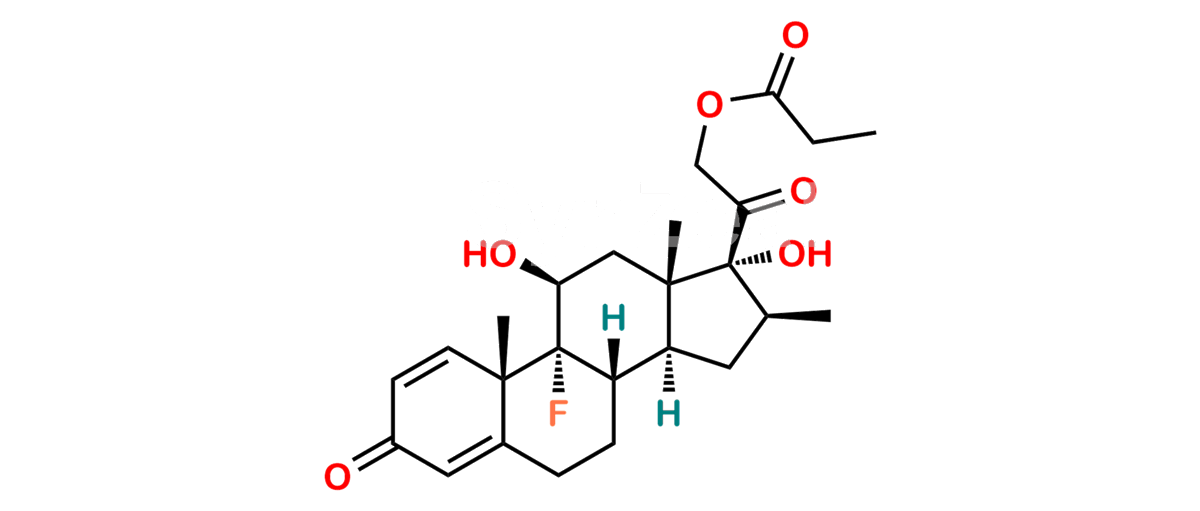
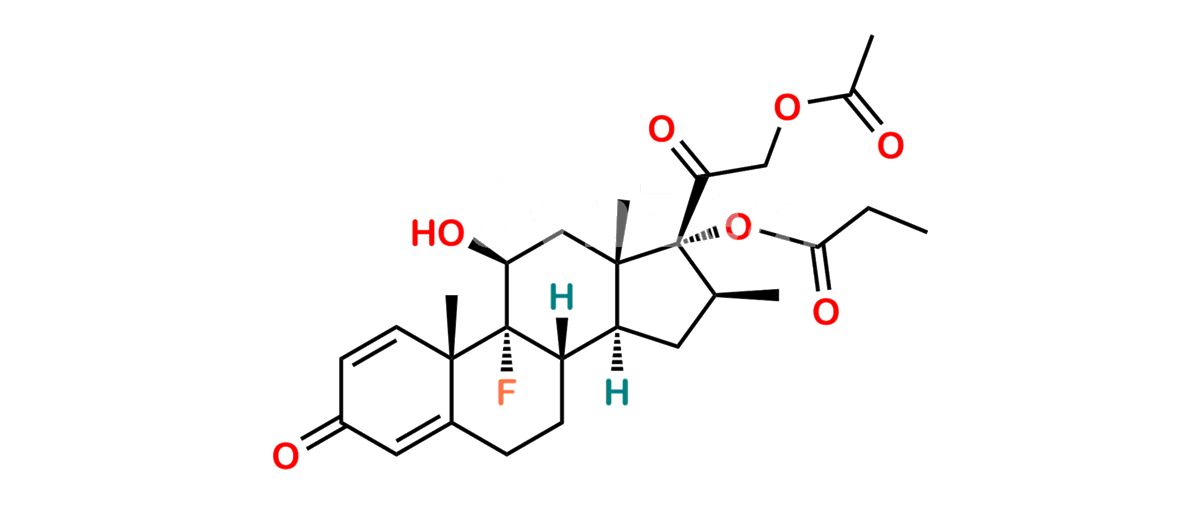
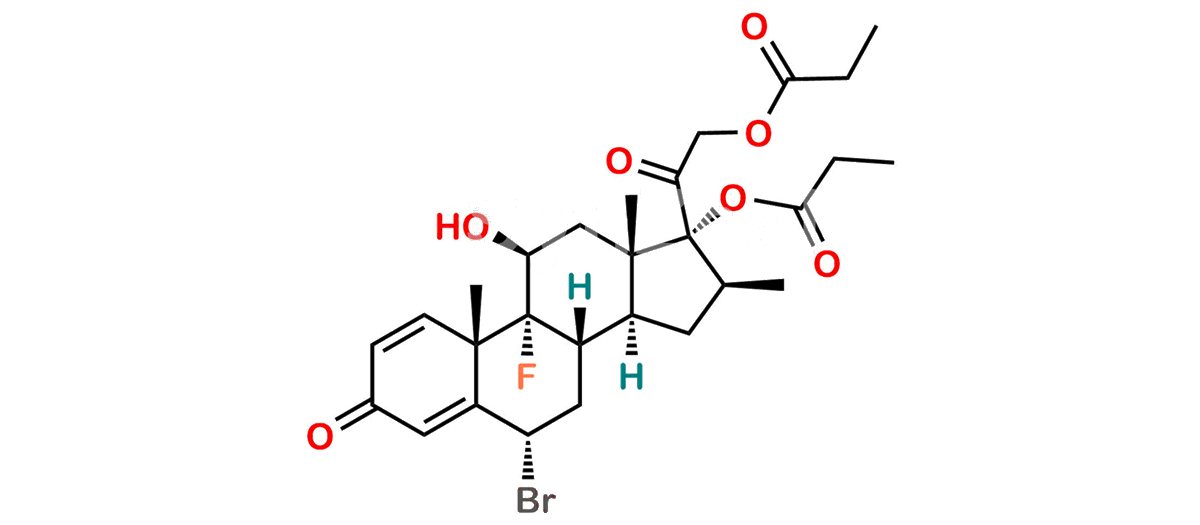
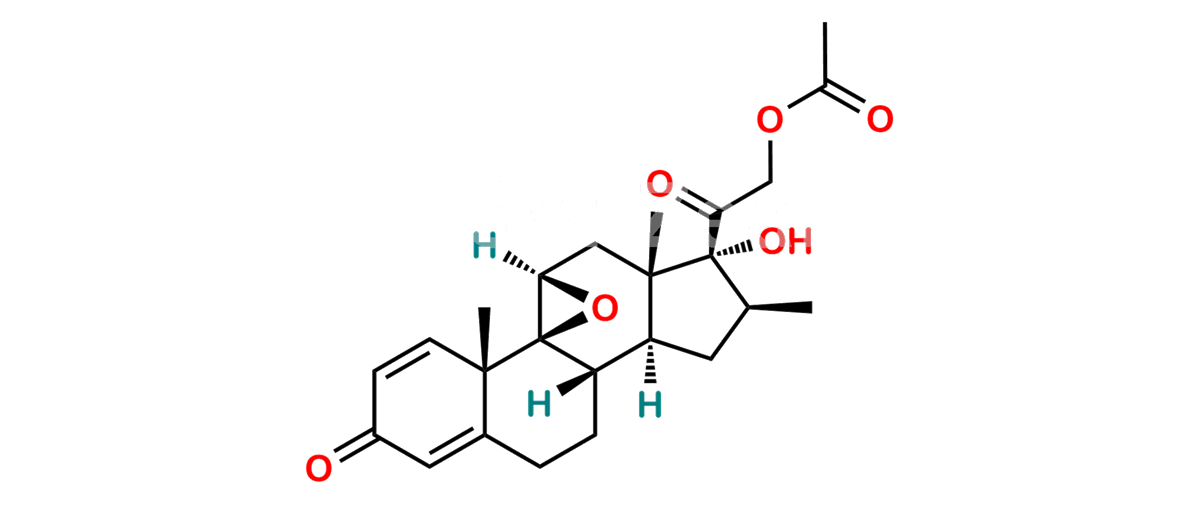
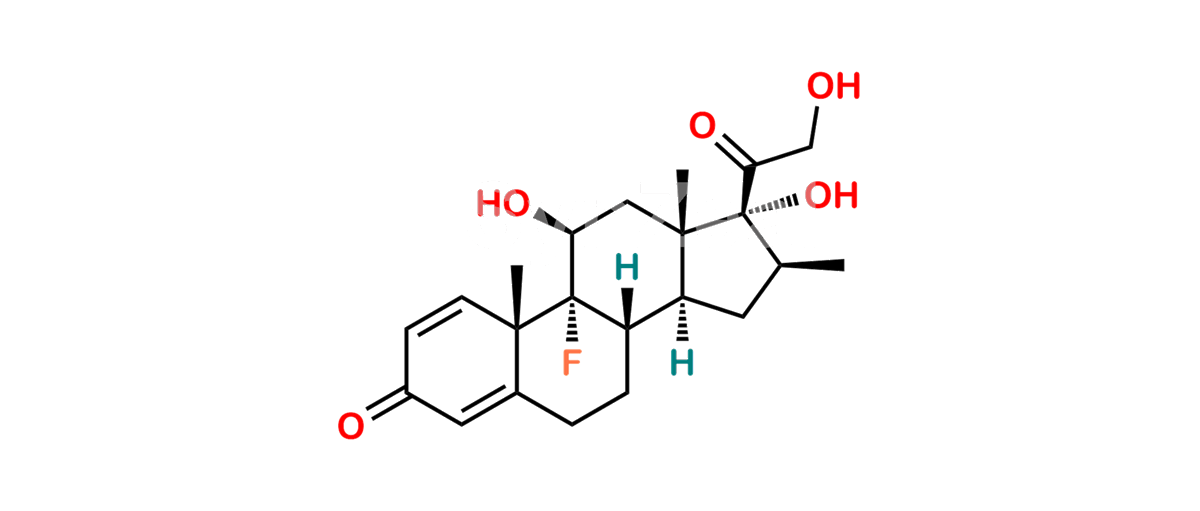
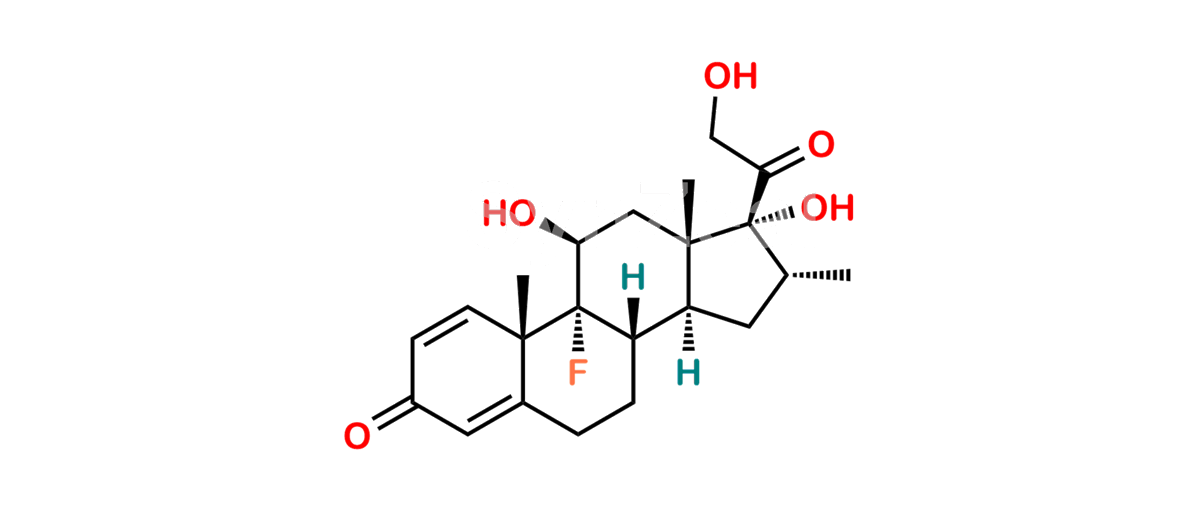
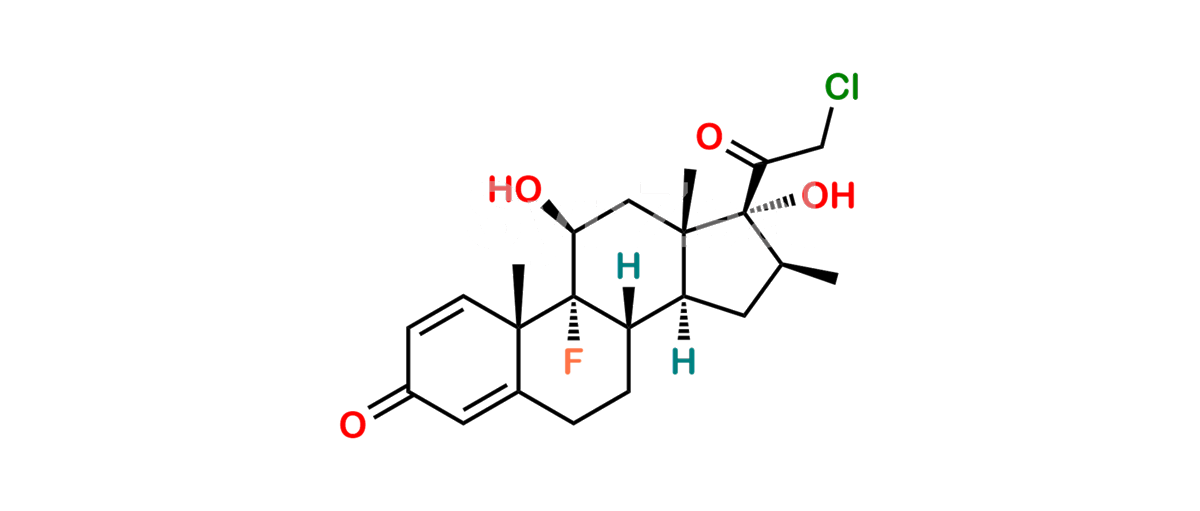
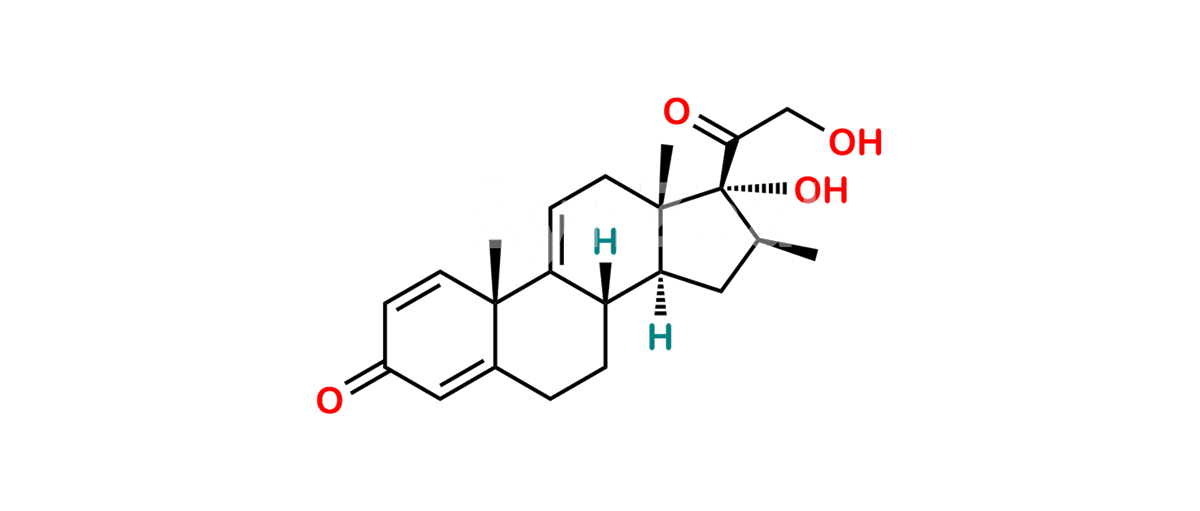
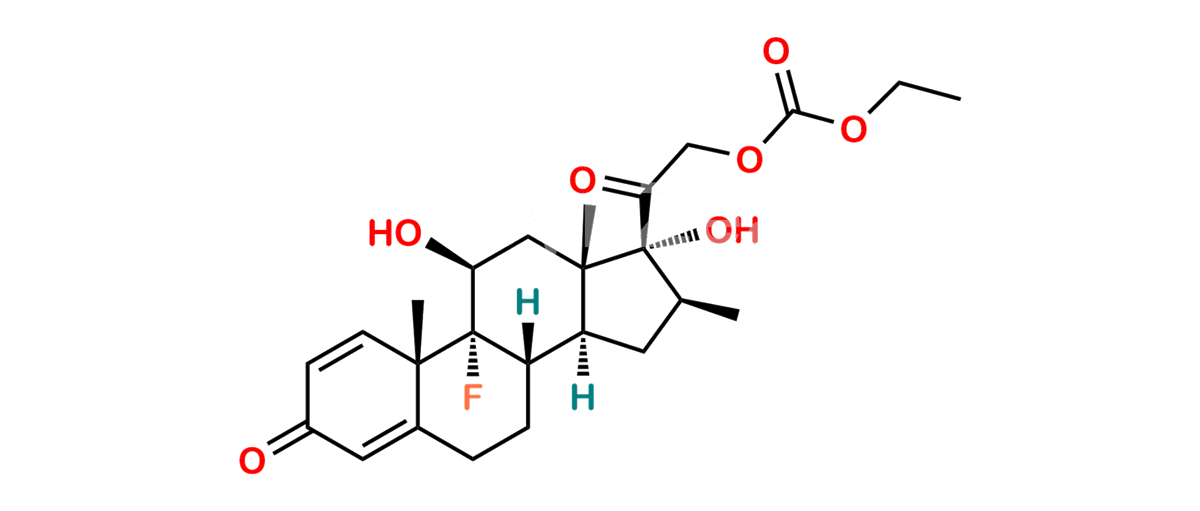
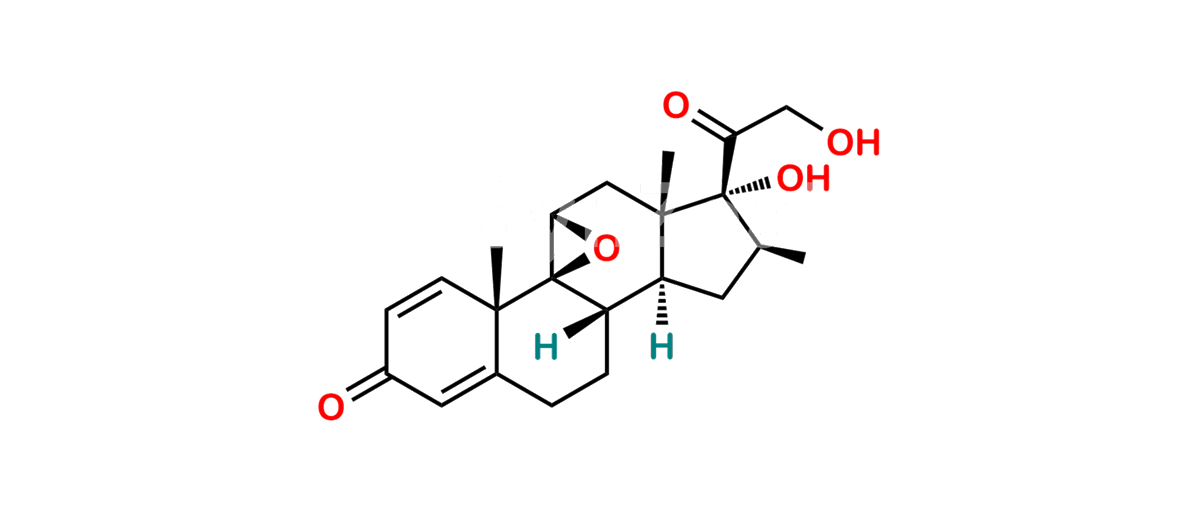
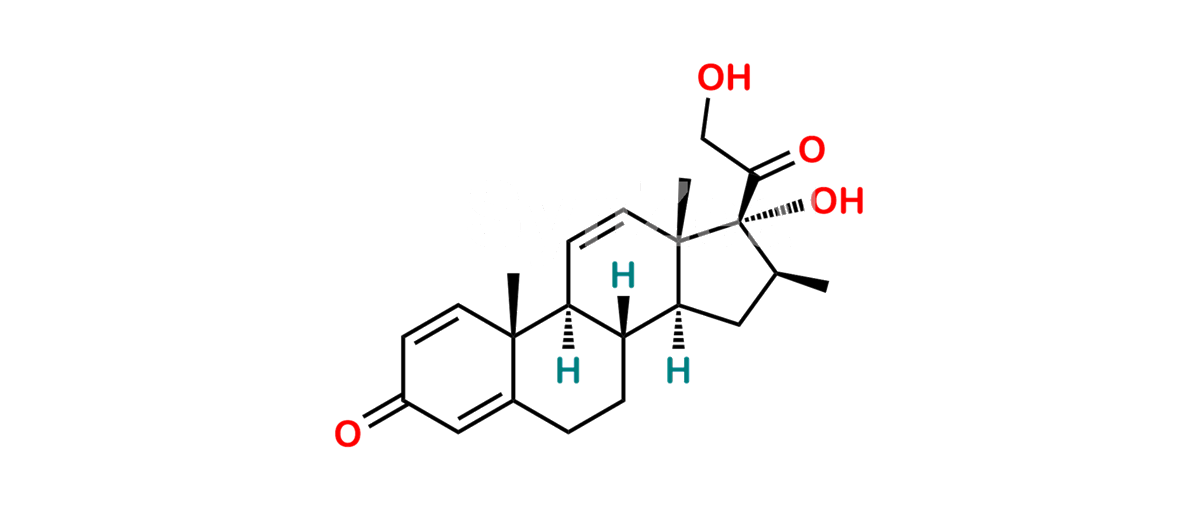
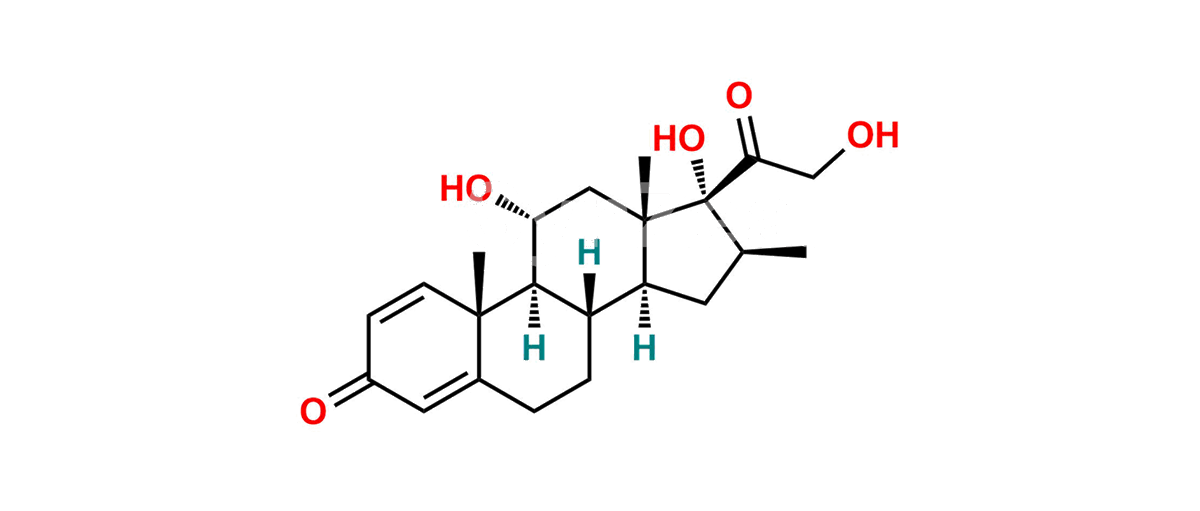
Betamethasone Impurity 19
Product Description
CAT No.
ALN-B025089
CAS No.
NA
Mol. F.
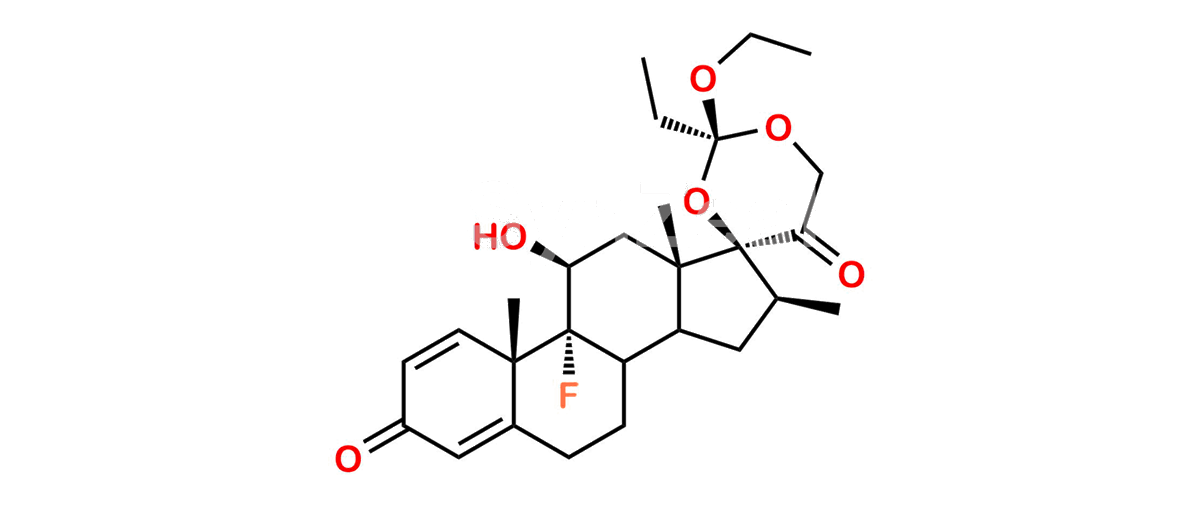
C27H37FO6
Mol. Wt.
476.6
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
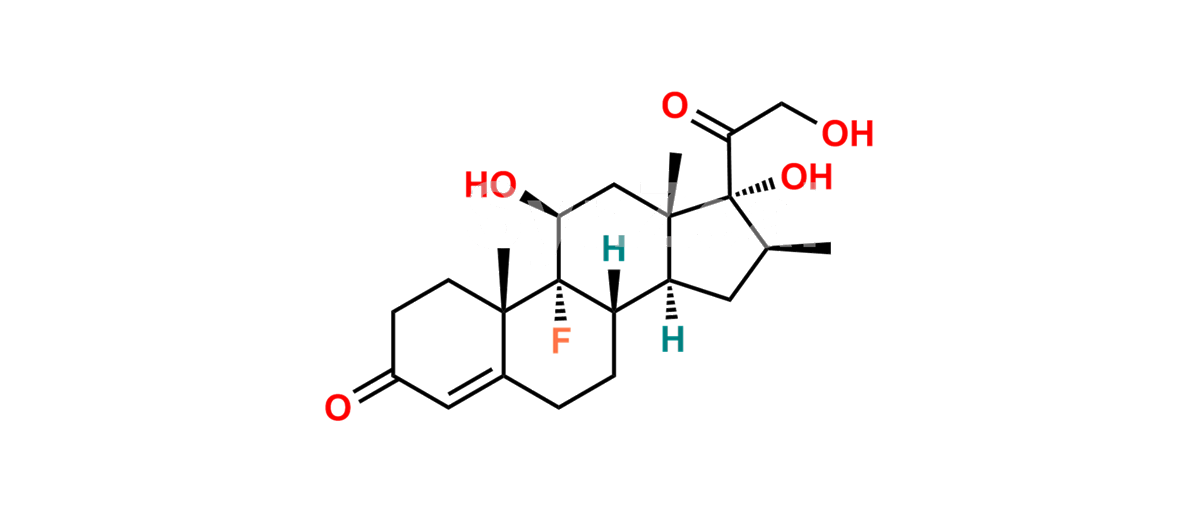
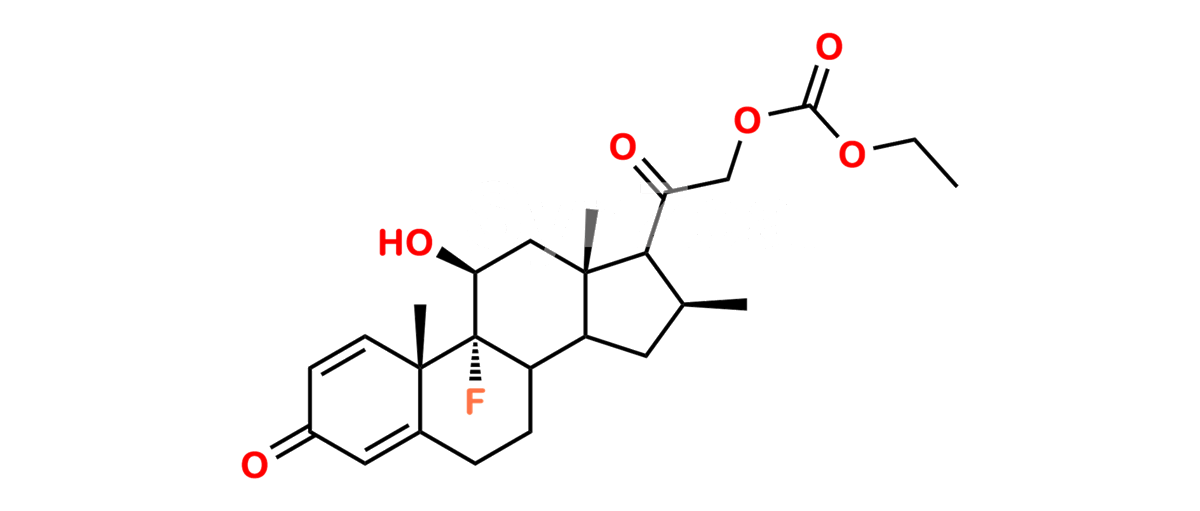
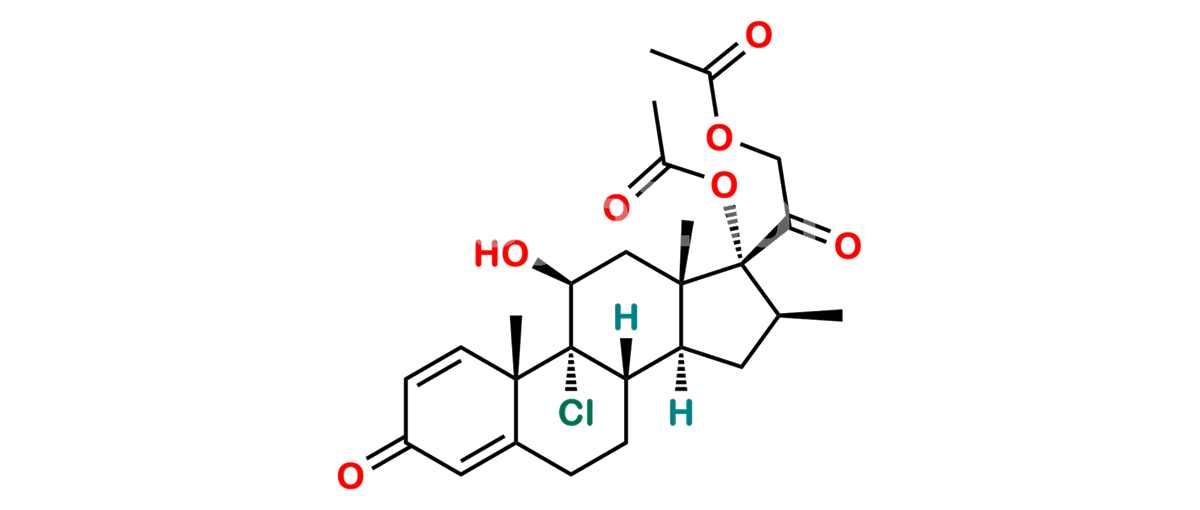
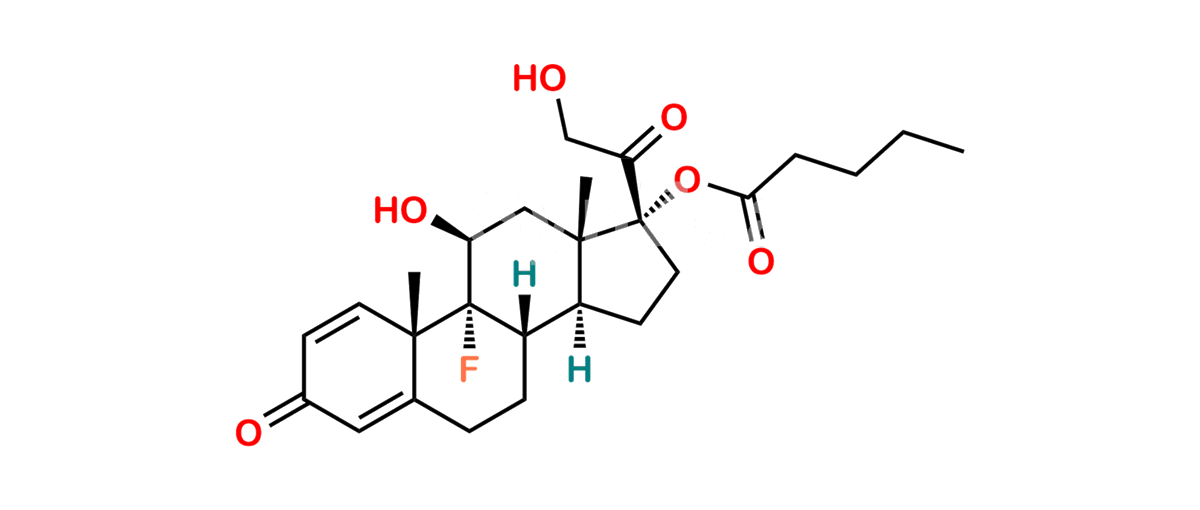
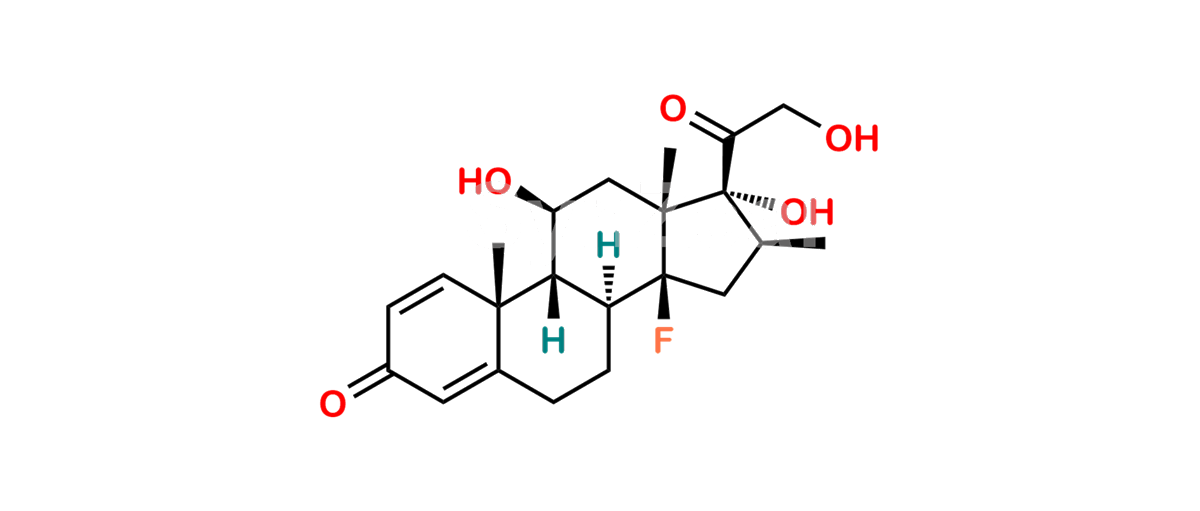
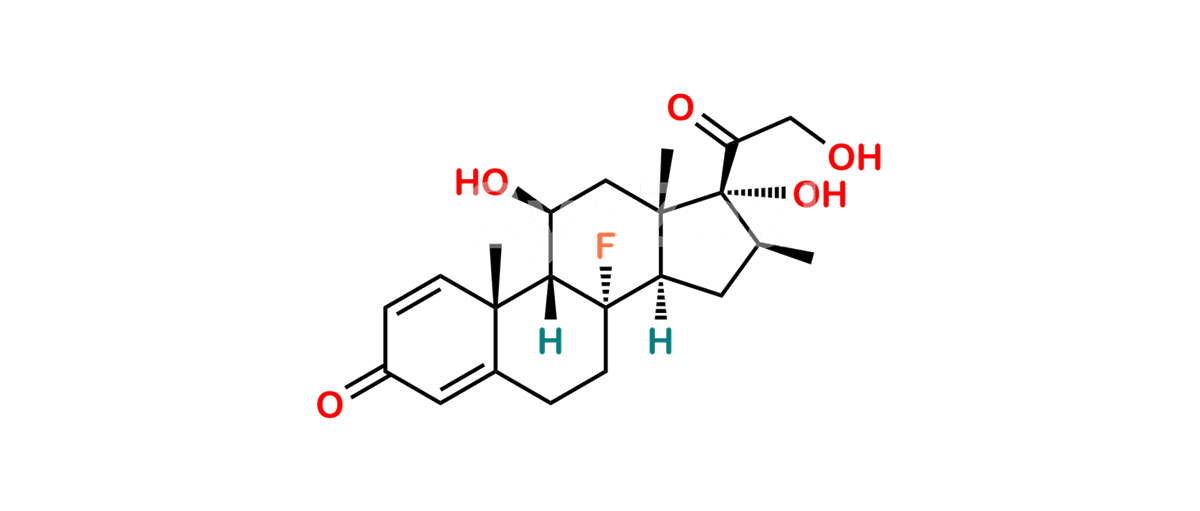
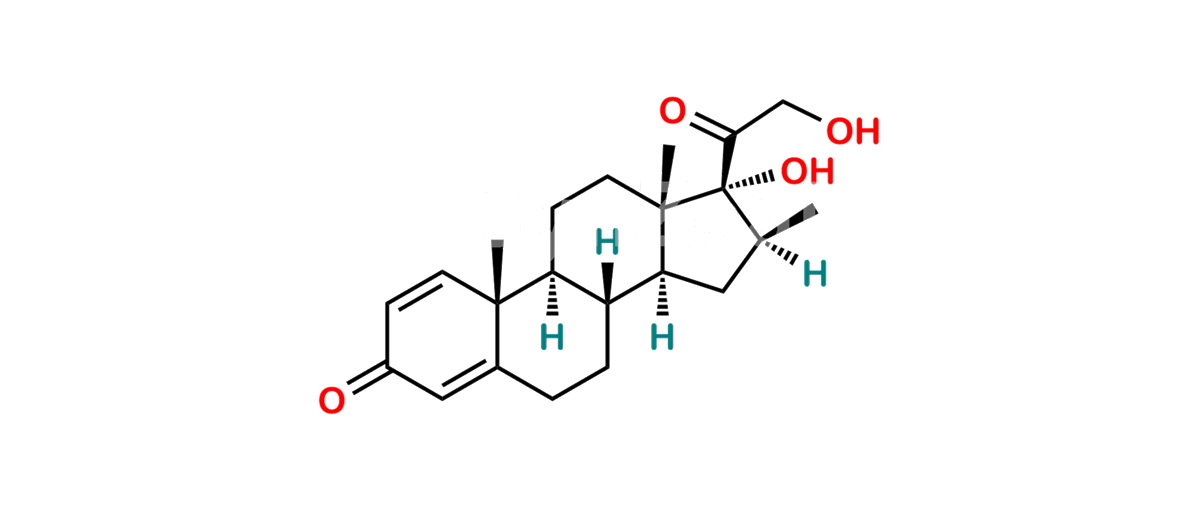
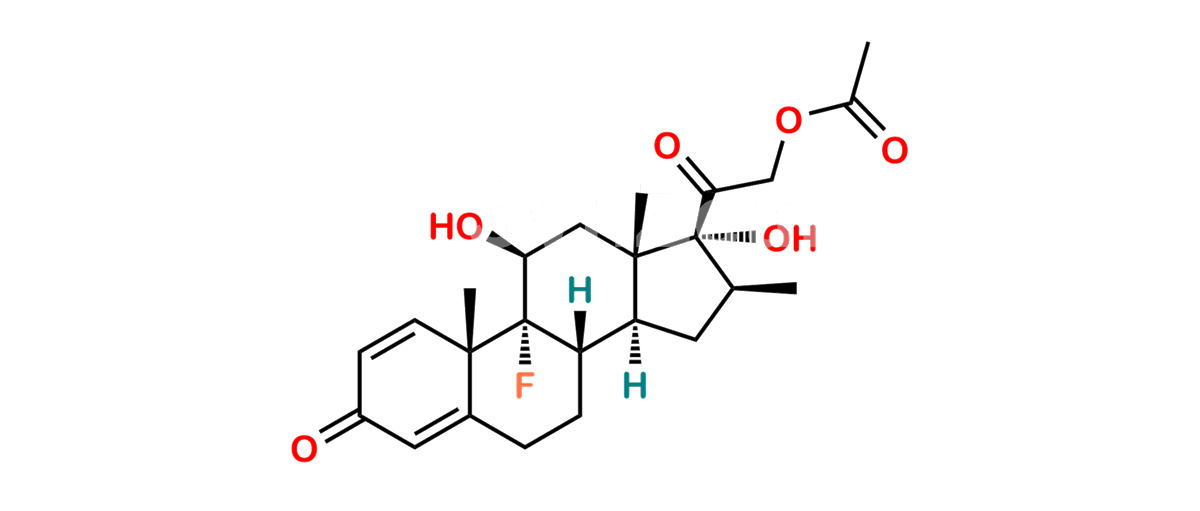
Chemical Name : (2’S,9R,10S,11S,13S,16S,17S)-2′-Ethoxy-2′-ethyl-9-fluoro-11-hydroxy-10,13,16-trimethyl-7,8,9,10,11,12,13,14,15,16-decahydrospiro[cyclopenta[a]phenanthrene-17,4′-[1,3]dioxane]-3,5′(6H)-dione
Smiles : O=C1C=C[C@]2(C)[C@@]3(F)[C@@H](O)C[C@]4(C)[C@@]5(O[C@@](CC)(OCC)OCC5=O)[C@@H](C)CC4C3CCC2=C1
Technical Data
Reference
Application of LCu2013MSn in conjunction with mechanism-based stress studies in the elucidation of drug impurity structure: Rapid identification of a process impurity in betamethasone 17-valerate drug substance
Min Li, Mingxiang Lin, Abu RustumnJournal of Pharmaceutical and Biomedical Analysis 48 (2008) 1451u20131456
Development and Validation of Stability-indicating HPLC Method for Betamethoasone Dipropionate and Related Substances in Topical Formulation
A. s. vairale
, p. sivaswaroop1 and s. bandana – Indian Journal of Pharmaceutical Sciences 74(2):107-15
RFQ