No products in the cart.
Daclatasvir Impurity 7

Product Description
CAT No.
ALN-D036029
CAS No.
4072-67-7
Mol. F.
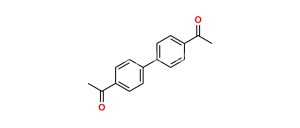
C16H12Br2O2
Mol. Wt.
396.1
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
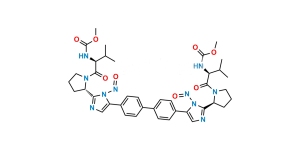
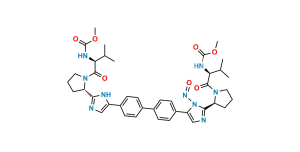
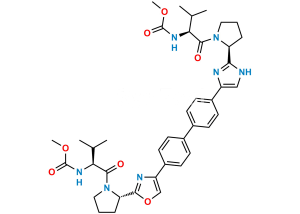
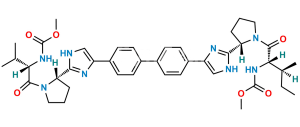
Chemical Name : 1,1′-([1,1′-Biphenyl]-4,4′-diyl)bis(2-bromoethan-1-one)
Smiles : BrCC(C1=CC=C(C2=CC=C(C(CBr)=O)C=C2)C=C1)=O
Inchi : InChI=1S/C40H50N8O6.H/c1-23(2)33(45-39(51)53-5)37(49)47-19-7-9-31(47)35-41-21-29(43-35)27-15-11-25(12-16-27)26-13-17-28(18-14-26)30-22-42-36(44-30)32-10-8-20-48(32)38(50)34(24(3)4)46-40(52)54-6;/h11-18,21-24,31-34H,7-10,19-20H2,1-6H3,(H,41,43)(H,42,44)(H,45,51)(H,46,52);/t31-,32-,33-,34-;/m0./s1/i;1+1
Technical Data
Reference
A validated stability-indicating reverse-phase high-performance liquid chromatography method for daclatasvir, identification and characterization of degradation products using LC-ESI-QTOF-MS
By Warghade, Snehal V.; Bothara, Kailash G.nFrom Asian Journal of Pharmaceutical and Clinical Research (2019), 12(5), 302-308
Development and validation of HPLC fluorescence and UPLC/DAD stability-indicating methods for determination of hepatitis C antiviral agent daclatasvir
By Kamal, Andra H.; Ismail, Nahla S.; Mabroijk, Mokhtar M.; Bebawy, Lories I.; Mekky, Mai A. – From Journal of AOAC International (2019), 102(4), 1125-1131
A stability-indicating UPLC method for the determination of potential impurities and its mass by a new QDa mass detector in daclatasvir drug used to treat hepatitis C infection
By Jagadabi, Varaprasad; Kumar, P. V. Nagendra; Mahesh, Kasthuri; Pamidi, Srinivasu; Ramaprasad, L. A.; Nagaraju, D. – From Journal of Chromatographic Science (2019), 57(1), 44-53
RFQ