No products in the cart.
Daclatasvir Impurity 11

Product Description
CAT No.
ALN-D036046
CAS No.
135-73-9
Mol. F.
C14H11BrO
Mol. Wt.
275.1
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
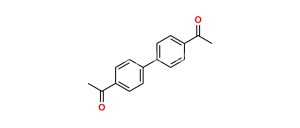
Chemical Name : 1-([1,1′-Biphenyl]-4-yl)-2-bromoethan-1-one
Smiles : O=C(C1=CC=C(C2=CC=CC=C2)C=C1)CBr
Technical Data
Reference
A validated stability-indicating reverse-phase high-performance liquid chromatography method for daclatasvir, identification and characterization of degradation products using LC-ESI-QTOF-MS
By Warghade, Snehal V.; Bothara, Kailash G.nFrom Asian Journal of Pharmaceutical and Clinical Research (2019), 12(5), 302-308
Development and validation of HPLC fluorescence and UPLC/DAD stability-indicating methods for determination of hepatitis C antiviral agent daclatasvir
By Kamal, Andra H.; Ismail, Nahla S.; Mabroijk, Mokhtar M.; Bebawy, Lories I.; Mekky, Mai A. – From Journal of AOAC International (2019), 102(4), 1125-1131
A stability-indicating UPLC method for the determination of potential impurities and its mass by a new QDa mass detector in daclatasvir drug used to treat hepatitis C infection
By Jagadabi, Varaprasad; Kumar, P. V. Nagendra; Mahesh, Kasthuri; Pamidi, Srinivasu; Ramaprasad, L. A.; Nagaraju, D. – From Journal of Chromatographic Science (2019), 57(1), 44-53
RFQ