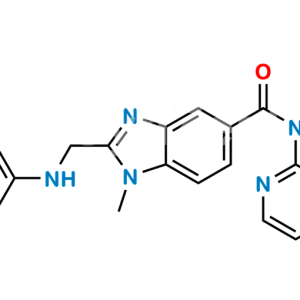
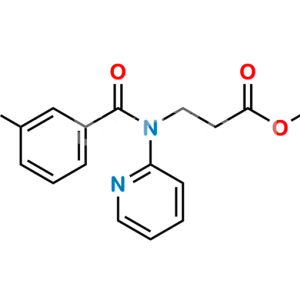
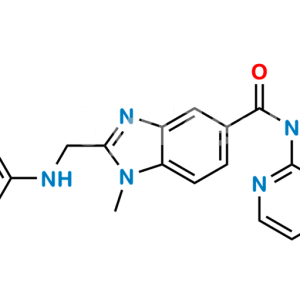
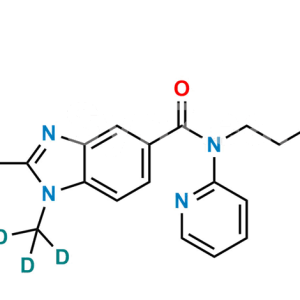
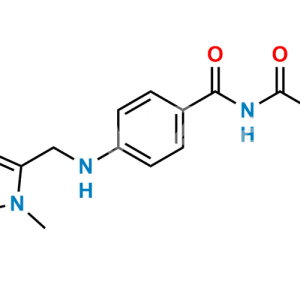
Dabigatran Impurity 50

Product Description
CAT No.
ALN-D012027
CAS No.
NA
Mol. F.
C29H33N7O3
Mol. Wt.
527.6
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
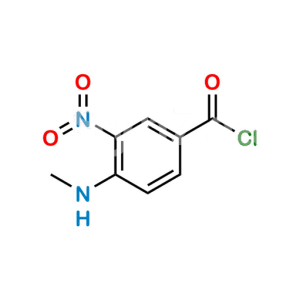
Chemical Name : Hexyl (Z)-(amino(4-(((1-methyl-5-(pyridin-2-ylcarbamoyl)-1H-benzo[d]imidazol-2-yl)methyl)amino)phenyl)methylene)carbamate
Smiles : O=C(C1=CC=C(N(C)C(CNC2=CC=C(/C(N)=N/C(OCCCCCC)=O)C=C2)=N3)C3=C1)NC4=NC=CC=C4
Inchi : InChI=1S/C12H9NO2S/c14-13(15)11-8-4-5-9-12(11)16-10-6-2-1-3-7-10/h1-9H
Technical Data
Reference
Novel method for separation and quantification of potential impurities by RP-HPLC from KSM stage to API stage of dabigatran mesylate
By Jhansi, T. Naga; Nagaraju, R.; Kumar, D. Jaya Deep; Rao, G. NageswaranFrom International Journal of Pharmaceutical Sciences and Research (2021), 12(3), 1762-1779
Development and Validation of RP-UPLC Method for the Determination of Process and Degradant Impurities Present in Dabigatran Etexilate Mesylate Capsules Using High Strength Silica-T3 Sorbent Column
By Mantena, Bhaskara P. V.; Rao, Sumathi V.; Suryakala, D.; Ramakrishna, K.; Srikanth Reddy, R. – From Analytical Chemistry Letters (2016), 6(5), 595-611
Determination of dabigatran etexilate mesylate by HPLC
By Jia, Ling-xiao; Tang, Lei; Zhang, Shu-ting; Song, Rui-zhi; Gao, Zhan-wen – From Shipin Yu Yaopin (2015), 17(3), 195-197
RFQ