No products in the cart.
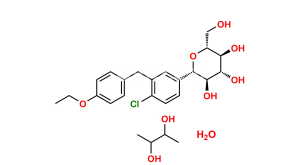
Dapagliflozin Propanediol Monohydrate

Product Description
CAT No.
ALN-D015045
CAS No.
960404-48-2
Mol. F.
C21H25ClO6 : C3H8O2 : H2O
Mol. Wt.
408.9 : 76.1 : 18.0
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
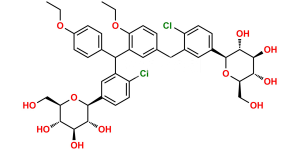
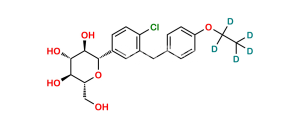
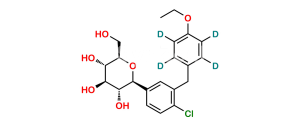
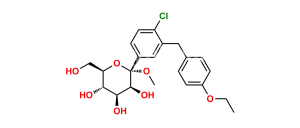
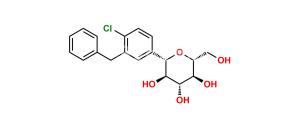
Chemical Name : (2S,3R,4R,5S,6R)-2-(4-Chloro-3-(4-ethoxybenzyl)phenyl)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol compound with (S)-propane-1,2-diol (1:1) hydrate
Smiles : O[C@H]1[C@H](C2=CC(CC3=CC=C(OCC)C=C3)=C(Cl)C=C2)O[C@H](CO)[C@@H](O)[C@@H]1O.C[C@H](O)CO.O
Inchi : InChI=1S/C26H27NO9.ClH/c1-10-21(29)15(27)7-17(35-10)36-16-9-26(34,11(2)28)8-14-18(16)25(33)20-19(24(14)32)22(30)12-5-3-4-6-13(12)23(20)31;/h3-6,10,15-17,21,29,32-34H,7-9,27H2,1-2H3;1H/t10-,15-,16-,17+,21+,26-;/m0./s1
Technical Data
Reference
Stability indicating rp-hplc method for simultaneous estimation of dapagliflozin and saxagliptin in pharmaceutical dosage forms
By Thota, Hanisha; Soundarya, G.nFrom Journal of Global Trends in Pharmaceutical Sciences (2021), 12(1), 9131-9137
Method development and validation of a stability-indicating reversed-phase liquid chromatographic method for the simultaneous estimation of metformin and dapagliflozin in presence of their degradation products
By Kotecha, Nidhi; Patel, Jayvadan – From International Journal of Pharmaceutical Sciences Review and Research (2019), 56(2), 1-6.
Validation of a newly developed stability indicating RP-liquid chromatographic method for the quantitative determination of dapagliflozin
By Sura, Sreenivasulu; Modalavalasa, Rameswara Rao; Kothapalli, ChandraSekhar B. – From Pharma Chemica (2018), 10(1), 93-102
RFQ