No products in the cart.
Domperidone EP Impurity F
Product Description
CAT No.
ALN-D002007
CAS No.
NA
Mol. F.
C37H42Cl2N8O3 : 2(C2HF3O2)
Mol. Wt.
717.7 : 2(114.0)
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
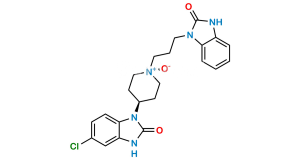
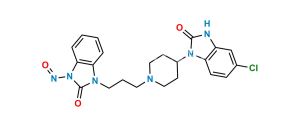
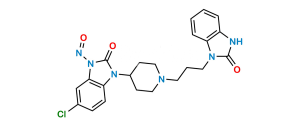
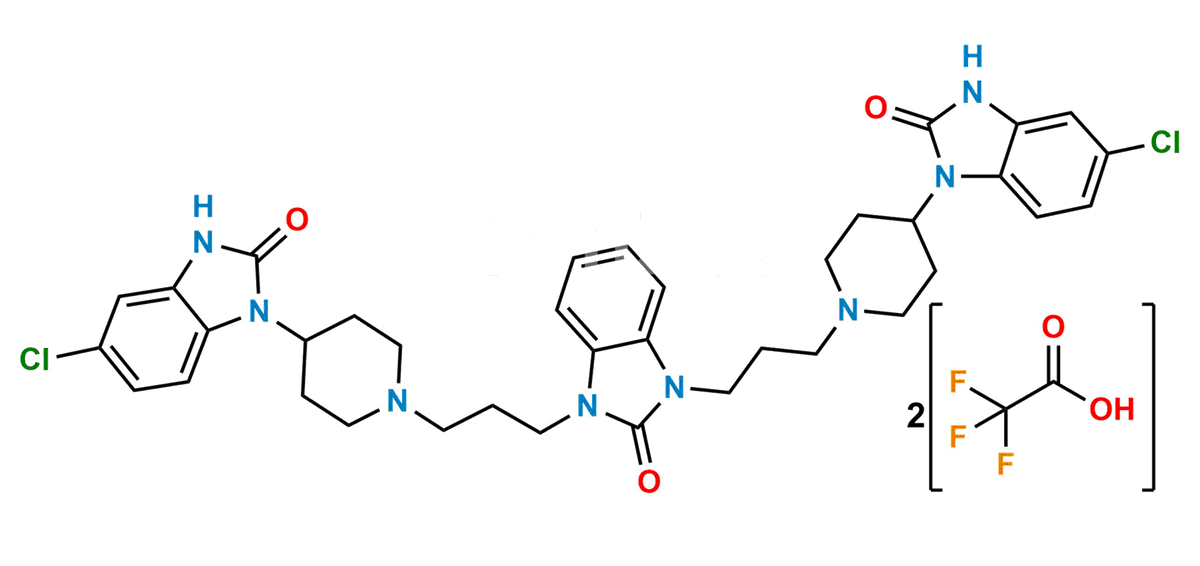
Chemical Name : 1,3-Bis[3-[4-(5-chloro-2-oxo-2,3-dihydro-1H-benzimidazol-1-yl)piperidin-1-yl]propyl]-1,3-dihydro-2H-benzimidazol-2-one bis(2,2,2-trifluoroacetate)
Smiles : O=C1N(C2CCN(CCCN3C4=CC=CC=C4N(CCCN5CCC(N6C7=CC=C(Cl)C=C7NC6=O)CC5)C3=O)CC2)C8=CC=C(Cl)C=C8N1.O=C(O)C(F)(F)F
Inchi : InChI=1S/C32H34ClN7O3/c33-22-11-12-27-25(21-22)35-31(42)40(27)23-13-19-36(20-14-23)15-5-17-38-28-9-3-4-10-29(28)39(32(38)43)18-6-16-37-26-8-2-1-7-24(26)34-30(37)41/h1-4,7-12,21,23H,5-6,13-20H2,(H,34,41)(H,35,42)
Technical Data
Reference
Stability indicating simultaneous determination of domperidone (DP), methylparaben (MP) and propylparaben by high performance liquid chromatography (HPLC)
Mohammed Shahid Ali u2217, Mohsin Ghori, Aamer Roshanali KhatrinJournal of Pharmaceutical and Biomedical Analysis 41 (2006) 358u2013365
The Ultra-Performance Liquid Chromatography Determination of Domperidone and Its Process-Related Impurities
Laura Curtin Whelan, Michael Geary, Mary Wharton, Paul Sweetman – Journal of Chromatographic Science, Volume 53, Issue 2, February 2015, Pages 226–232
A Single Gradient Stability-Indicating Reversed-Phase LC Method for the Estimation of Impurities in Omeprazole and Domperidone Capsules
Raja Kumar Seshadri,
RFQ