No products in the cart.
Erlotinib Impurity F
Product Description
CAT No.
ALN-E018068
CAS No.
1809951-10-7
Mol. F.
C28H34N4O9
Mol. Wt.
570.6
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
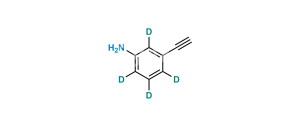
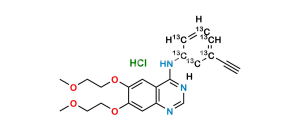
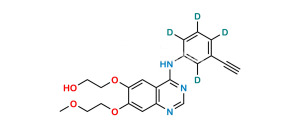
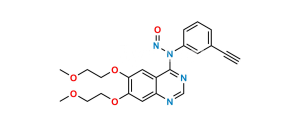
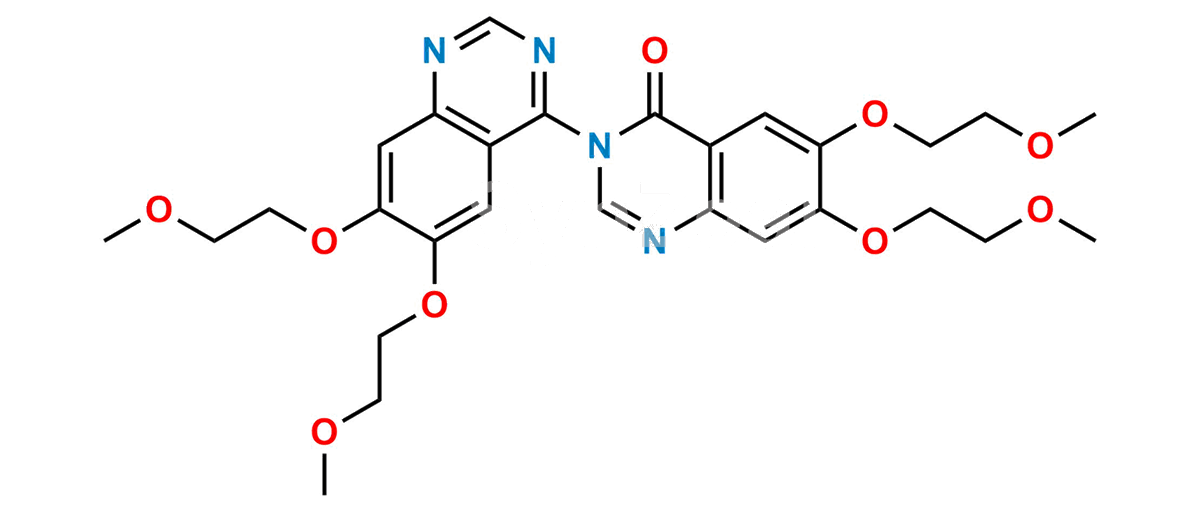
Chemical Name : 6,6′,7,7′-tetrakis(2-methoxyethoxy)-4H-[3,4′-biquinazolin]-4-one
Smiles : COCCOC1=CC2=C(C(N3C=NC(C=C(OCCOC)C(OCCOC)=C4)=C4C3=O)=NC=N2)C=C1OCCOC
Inchi : InChI=1S/C27H40O6/c1-4-5-6-7-23(31)33-16-22(30)27(32)13-11-20-19-9-8-17-14-18(28)10-12-25(17,2)24(19)21(29)15-26(20,27)3/h14,19-21,24,29,32H,4-13,15-16H2,1-3H3/t19-,20-,21-,24+,25-,26-,27-/m0/s1
Technical Data
Reference
Erlotinib in combination with capecitabine and docetaxel in patients with metastatic breast cancer: A dose-escalation study
Chris Twelvesa, Joseu00b4 M. Trigob , Rob Jonesc , Flavio De Rosad , Ashok Rakhite , Scott Fettnere , Tonya Wrightc , Joseu00b4 BaselganEuropean journal of cancer 44 (2008) 419 u2013 426
Development and validation of a stability indicating HPLC method for the quantification of impurities in Erlotinib hydrochloride dosage forms
BabuC1, Narasimha\xa0 RaoK.L1,\xa0 DevannaN1, Suresh Reddy\xa0 K.V.N – Solubility Measurement and Correlation of Two Erlotinib Hydrochloride Polymorphs in Alcohols and Ketones from 273.15 to 323.15 K
Jinghuan Zhai, Junrui Zhao, Zhenzhen Chen, Changwei Xiao, Xijian Liu, Lijuan Zhang, and Jie Lu
J. Chem. Eng. Data 2017, 62, 1, 516–524 – Separation and Determination of Process-Related Impurities of Erlotinib Using Reverse-Phase HPLC with a Photo-Diode Array Detector
RFQ