No products in the cart.
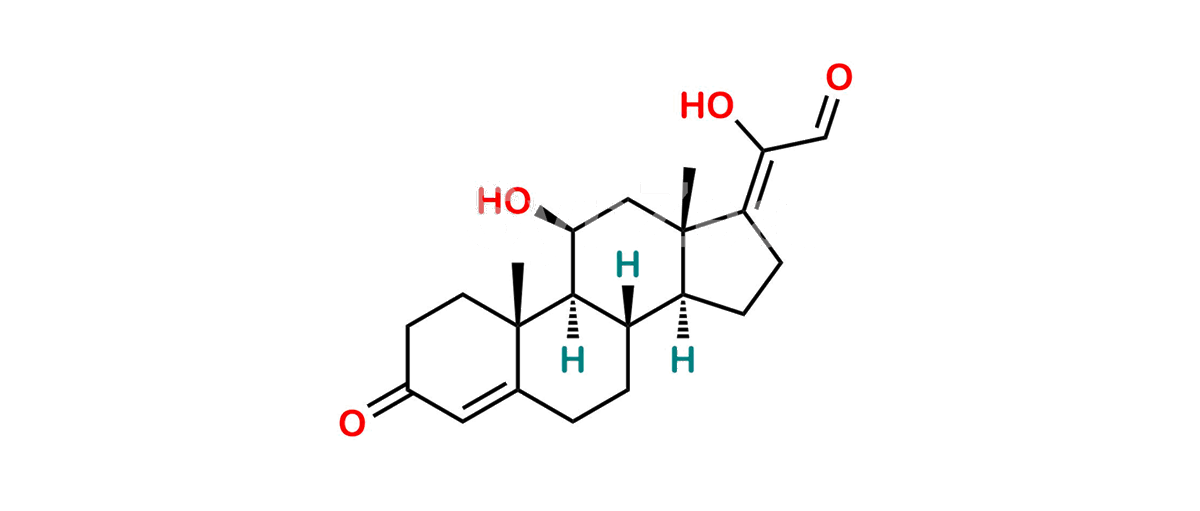
Hydrocortisone Impurity C
Product Description
CAT No.
ALN-H008025
CAS No.
105562-13-8
Mol. F.
C21H28O4
Mol. Wt.
344.5
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
Chemical Name : (11 ,17Z)-11,20-Dihydroxy-3-oxo-pregna-4,17(20)-dien-21-al
Smiles : C[C@@](C(CC1)=CC2=O)(CC2)[C@]3([H])[C@]1([H])[C@@](CC/C4=C(O)\C=O)([H])[C@]4(C)C[C@@H]3O
Inchi : InChI=1S/C21H28O4/c1-20-8-7-13(23)9-12(20)3-4-14-15-5-6-16(18(25)11-22)21(15,2)10-17(24)19(14)20/h9,11,14-15,17,19,24-25H,3-8,10H2,1-2H3/b18-16+/t14-,15-,17-,19+,20-,21-/m0/s1
Synonym : (Z)-17-deoxyaldehyde derivative
Technical Data
Reference
Comparative study of two different chromatographic approaches for quantitation of hydrocortisone acetate and pramoxine hydrochloride in presence of their impurities
Fawzia Ibrahim a , Asmaa Kamal El-Deen a,b , Kuniyoshi ShimizunJournal of Food and Drug Analysis Volume 26, Issue 3, July 2018, Pages 1160-1170
Novel stability indicating UHPLC method development and validation for simultaneous quantification of hydrocortisone acetate, pramoxine hydrochloride, potassium sorbate and sorbic acid in topical cream formulation
Lakshmi Narasimha RaoKatakamaThirupathiDongalabSanthosh KumarEttaboina – Talanta Open Volume 1, August 2020, 100004
Novel eco-friendly chromatographic determinations of hydrocortisone acetate, fusidic acid, their pharmacologically active impurities and pharmaceutical excipients: a comparative study
Maha M. Abdelrahman, Raghda Abdelmoneim Emam, Nouruddin W. Ali & Eglal A. Abdelaleem – Chemical Papers volume 74, pages2175–2187(2020)
RFQ