No products in the cart.
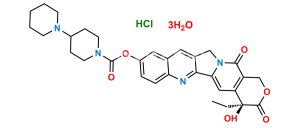
Irinotecan EP Impurity M (HCl salt)
Product Description
CAT No.
ALN-I010040
CAS No.
NA
Mol. F.
C33H38N4O7 : HCl
Mol. Wt.
602.7 : 36.5
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
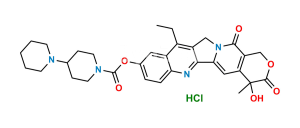
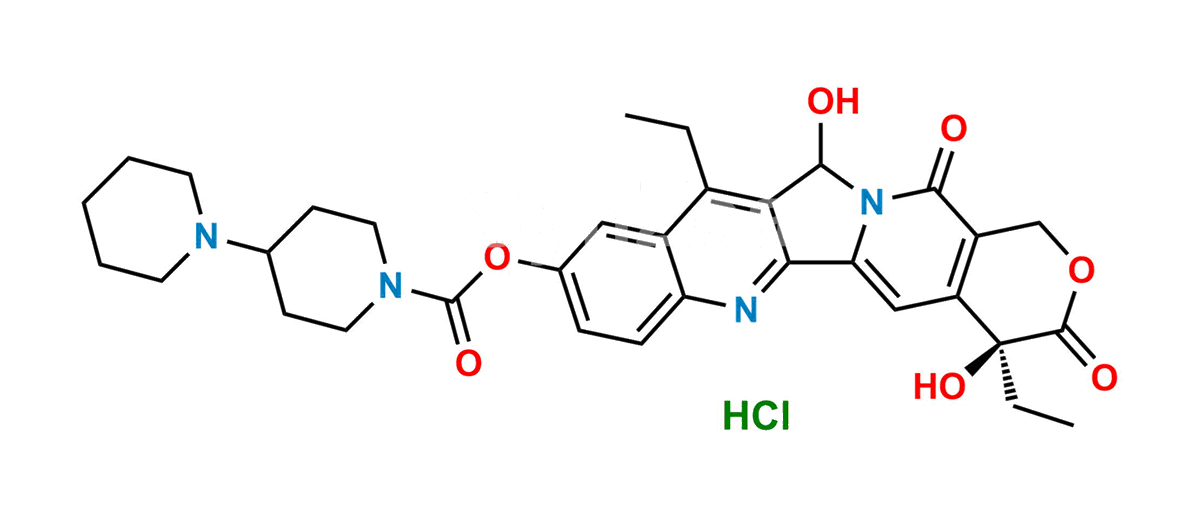
Chemical Name : (4S)-4,11-diethyl-4,12-dihydroxy-3,14-dioxo-3,4,12,14-tetrahydro-1H-pyrano[3′,4′:6,7]indolizino[1,2-b]quinolin-9-yl 1,4′-bipiperidine-1′-carboxylate hydrochloride
Smiles : OC(C(C1=NC2=CC=C(OC(N3CCC(N4CCCCC4)CC3)=O)C=C52)=C5CC)N6C1=CC([C@](O)7CC)=C(COC7=O)C6=O.Cl
Inchi : InChI=1S/C27H30N8O3/c1-6-26(36)29-21-15-22(25(38-5)16-24(21)33(2)13-14-35(4)32-37)31-27-28-12-11-20(30-27)19-17-34(3)23-10-8-7-9-18(19)23/h6-12,15-17H,1,13-14H2,2-5H3,(H,29,36)(H,28,30,31)
Synonym : 12-Hydroxy Irinotecan
Technical Data
Reference
RP-HPLC method development and validation for the simultaneous estimation of Irinotecan hydrochloride and Capecitabine in Active Pharmaceutical Ingredients (APIs)
By Vijaya Jyothi, M.; Bhargav, E.; Keerthana, B.; Varalakshmi, Devi K.nFrom International Journal of Research in Pharmaceutical Sciences (Madurai, India) (2018), 9(1), 63-67
Method development, validation and forced degradation studies of Irinotecan in bulk and pharmaceutical dosage form
By Swathi, Koduru; Farooqui, Tahmeena; Muntaha, Sidra Tul; Ayesha, Syeda Amtul; Shama, Syeda; Siddiqui, Sarah Imam – From World Journal of Pharmacy and Pharmaceutical Sciences (2018), 7(8), 996-1009
RFQ