No products in the cart.
Levofloxacin Impurity 5

Product Description
CAT No.
ALN-L020016
CAS No.
2489671-30-7
Mol. F.
C20H24FN3O4
Mol. Wt.
389.4
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
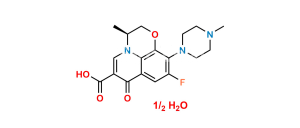
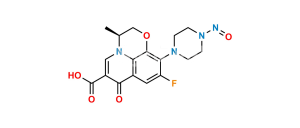
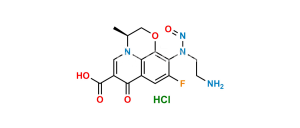
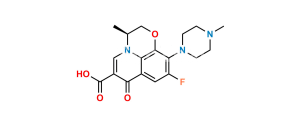
Chemical Name : ethyl (3R)-9-fluoro-3-methyl-10-(4-methylpiperazin-1-yl)-7-oxo-2,3-dihydro-7H-[1,4]oxazino[2,3,4-ij]quinoline-6-carboxylate
Smiles : CCOC(C1=CN(C2=C(C1=O)C=C3F)[C@@H](COC2=C3N4CCN(CC4)C)C)=O
Inchi : InChI=1S/C5H3N3O4/c9-7(10)4-1-5(8(11)12)3-6-2-4/h1-3H
Technical Data
Reference
In silico and in vitro genotoxicity evaluation of levofloxacin n-oxide, an impurity in levofloxacin
Qingfen Zhu1,2, Tao Li2 , Jun Li2 , Ming Guo2 , Weijian Wang2 , and Xiumei ZhangnToxicology Mechanisms and Methods, 2012; 22(3): 225u2013230
Identification, Isolation and Characterization of New Process-related Impurities in Levofloxacin
Kishor More
An integrated approach for detection and characterization of the trace impurities in levofloxacin using liquid chromatography– tandem mass spectrometry
, Leena Gupta, Brajesh Sharma, Prashant Patil, Dattatray Govare – Der Pharma Chemica, 2017, 9(12):7-13
RFQ