No products in the cart.
Metoprolol lactose Adduct Impurity

Product Description
CAT No.
ALN-M021033
CAS No.
NA
Mol. F.
C28H47NO11
Mol. Wt.
573.7
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
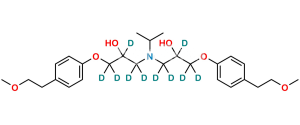
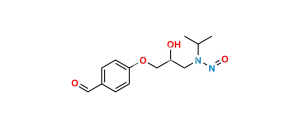
Chemical Name : (1S,2S,3S,4R,6R)-4-((5-hydroxy-5-((((S)-2-hydroxy-3-(4-(2-methoxyethyl)phenoxy)propyl)(isopropyl)amino)methyl)-2-(hydroxymethyl)tetrahydrofuran-3-yl)oxy)-6-(hydroxymethyl)cyclohexane-1,2,3-triol
Smiles : O[C@@H]1[C@H](O)[C@H](O)[C@H](OC2C(CO)OC(CN(C[C@H](O)COC3=CC=C(CCOC)C=C3)C(C)C)(O)C2)C[C@@H]1CO
Inchi : InChI=1/C13H13NO/c1-13(15,11-7-3-2-4-8-11)12-9-5-6-10-14-12/h2-10,15H,1H3/i2D,3D,4D,7D,8D
Technical Data
Reference
Identification of a new by-product detected in metoprolol tartrate
magnus erickson,$ karl-erik karlsson,$ bo lamm,u00a7 sam larsson,$ lars a. svensson$ and jorgen vessmannJournal of Pharmaceutical & Biomedical Analysis, Vol. 13, No. 4/5, pp. 567-574, 1995
An improved validated HPLC method for separation of metoprolol and hydrochlorothiazide impurities in metoprolol and hydrochlorothiazide tablets
Avinash S. Patil, Shakil S. Sait, Abhijit Deshamukh and Girish Deshpande – Der Pharmacia Lettre, 2015, 7 (2):183-190
Simultaneous Quantitative Determination of Metoprolol, Atorvastatin and Ramipril in Capsules by a Validated Stability-Indicating RP-UPLC Method
raja kumar seshadri
RFQ