No products in the cart.
Pazopanib Impurity 8

Product Description
CAT No.
ALN-P013033
CAS No.
NA
Mol. F.
C21H22N6O3S
Mol. Wt.
438.5
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
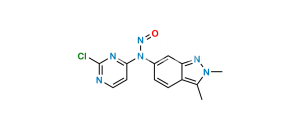
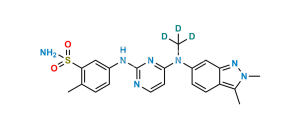
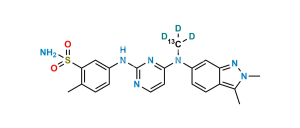
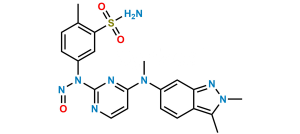
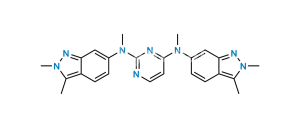
Chemical Name : 5-((4-((2,3-dimethyl-2H-indazol-6-yl)(methyl)amino)pyrimidin-2-yl)amino)-2-methylbenzenesulfonic acid
Smiles : CC1=CC=C(NC2=NC(N(C)C3=CC4=NN(C)C(C)=C4C=C3)=CC=N2)C=C1S(=O)(O)=O
Technical Data
Reference
Analytical control of process impurities in Pazopanib hydrochloride by impurity fate mapping
Yan Li 1, David Q Liu, Shawn Yang, Ravinder Sudini, Michael A McGuire, Dharmesh S Bhanushali, Alireza S KordnJ Pharm Biomed Anal. 2010 Aug 1;52(4):493-507.
Determination and characterization of process impurities in pazopanib hydrochloride drug substance
musty sharada, ravichandra babu – Int J Pharm Pharm Sci, Vol 8, Issue 4, 97-102
Characterization of forced degradation products of pazopanib hydrochloride by UHPLC?Q?TOF/MS and in silico toxicity prediction
Prinesh N. Patel\xa0 Pradipbhai D. Kalariya\xa0 Mahesh Sharma\xa0 Prabha Garg\xa0 M. V. N Kumar Talluri\xa0 S. Gananadhamu\xa0 R. Srinivas – Journal of mass spectrometry Volume50, Issue7 July 2015 Pages 918-928
RFQ