No products in the cart.
Prednisolone Sodium Phosphate USP Impurity D
Product Description
CAT No.
ALN-P027014
CAS No.
898-84-0
Mol. F.
C19H24O3
Mol. Wt.
300.4
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
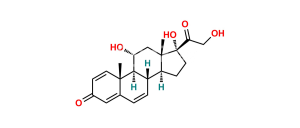
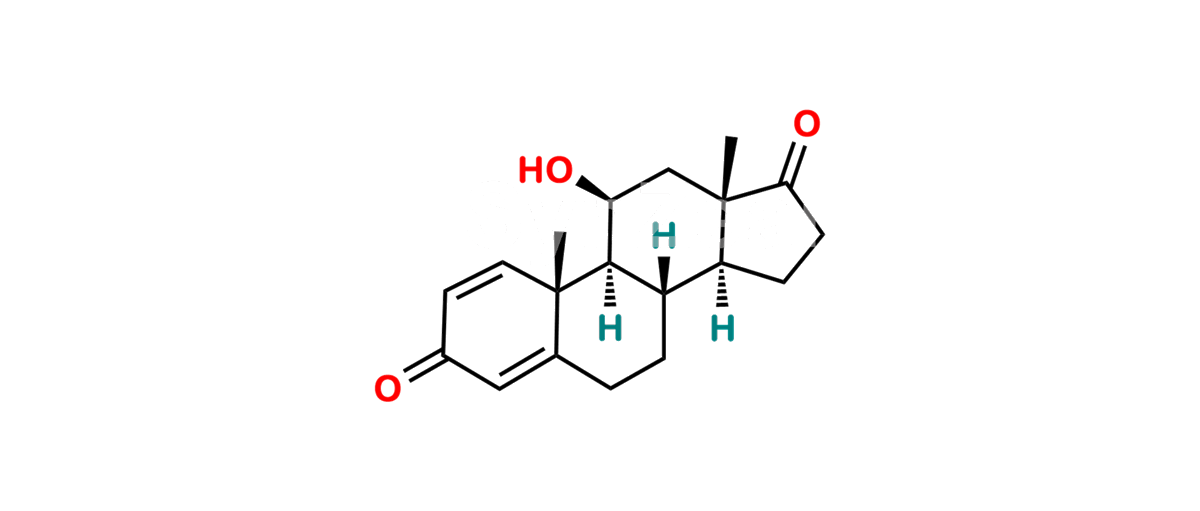
Chemical Name : 11β-Hydroxyandrosta-1,4-diene-3,17-dione (as per USP)
Smiles : C[C@@](C1=CC2=O)(C=C2)[C@]3([H])[C@](CC1)([H])[C@@](CCC4=O)([H])[C@]4(C)C[C@@H]3O
Inchi : InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29)
Synonym : 11β-Hydroxyboldione
Technical Data
Reference
Validated stability indicating RP-LC assay for determination of gatifloxacin and prednisolone acetatein ophthalmic preparations and biological samples
By Qadir, Muhammad A.; Shahzad, Shabnam; Ahmed, Mahmood; Razzaq, Syed S.; Shafiq, Muhmmad I.nFrom Latin American Journal of Pharmacy (2016), 35(5), 912-920
Novel stability indicating RP-HPLC method for the simultaneous estimation of moxifloxacin and prednisolone in bulk and their combined dosage form
By Potnuri, Naga Raju; Rao, G. Devala; Prasad, Y. Rajendra – From International Journal of Pharmaceutical Sciences and Research (2015), 6(5), 1965-1973
Development and validation of a new stability indicating reversed phase liquid chromatographic method for the determination of prednisolone acetate and impurities in an ophthalmic suspension
By Marley, Adrian; Stalcup, Apryll M.; Connolly, Damian – From Journal of Pharmaceutical and Biomedical Analysis (2015), 102, 261-266
RFQ