No products in the cart.
Ritonavir Impurity 14

Product Description
CAT No.
ALN-R003046
CAS No.
1797984-48-5
Mol. F.
C37H48N6O5S2
Mol. Wt.
720.9
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
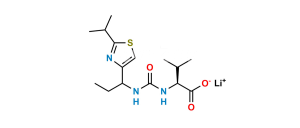
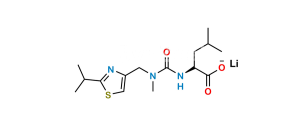
Chemical Name : [thiazol-5-ylmethyl ((2R,3S,5R)-3-hydroxy-5-((S)-2-(3-((2-isopropylthiazol-4-yl)methyl)-3-methylureido)-3-methylbutanamido)-1,6-diphenylhexan-2-yl)carbamate]
Smiles : O=C(OCC1=CN=CS1)N[C@@H]([C@@H](O)C[C@H](NC([C@@H](NC(N(CC2=CSC(C(C)C)=N2)C)=O)C(C)C)=O)CC3=CC=CC=C3)CC4=CC=CC=C4
Inchi : InChI=1S/C37H48N6O5S2/c1-24(2)33(42-36(46)43(5)20-29-22-49-35(40-29)25(3)4)34(45)39-28(16-26-12-8-6-9-13-26)18-32(44)31(17-27-14-10-7-11-15-27)41-37(47)48-21-30-19-38-23-50-30/h6-15,19,22-25,28,31-33,44H,16-18,20-21H2,1-5H3,(H,39,45)(H,41,47)(H,42,46)/t28-,31+,32+,33-/m0/s1
Technical Data
Reference
Development and validation of stability-indicating UPLC-TUV method for simultaneous estimation of darunavir and ritonavir in bulk and tablet dosage form
By Kamalakannan, Dhanabalan; AnandaThangadurai, SubramaniamnFrom International Journal of Research in Pharmaceutical Sciences (Madurai, India) (2021), 12(1), 611-619
Stability indicating method development and validation for simultaneous estimation of Lopinavir and Ritonavir by using RP-HPLC
By Raghu, P. S. – From World Journal of Pharmaceutical Research (2018), 7(3), 1750-1757
Stability-indicating RP-HPLC method for simultaneous quantification of ombitasvir, paritaprevir and ritonavir in tablet dosage form
By Kuna, Mangamma; Dannana, Gowri Sankar – From Asian Journal of Chemistry (2018), 30(6), 1277-1283
RFQ