No products in the cart.
Hydroxy Saxagliptin-13C,D2 Hydrochloride
Product Description
CAT No.
ALN-S005D08
CAS No.
1572922-53-2
Mol. F.
C1713CH23D2N3O3 : HCl
Mol. Wt.
334.4 : 36.5
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
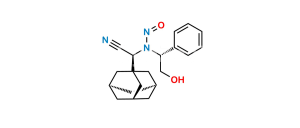
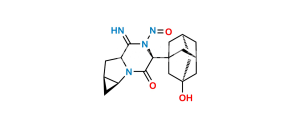
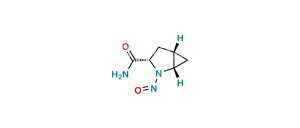
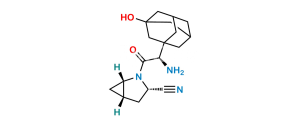
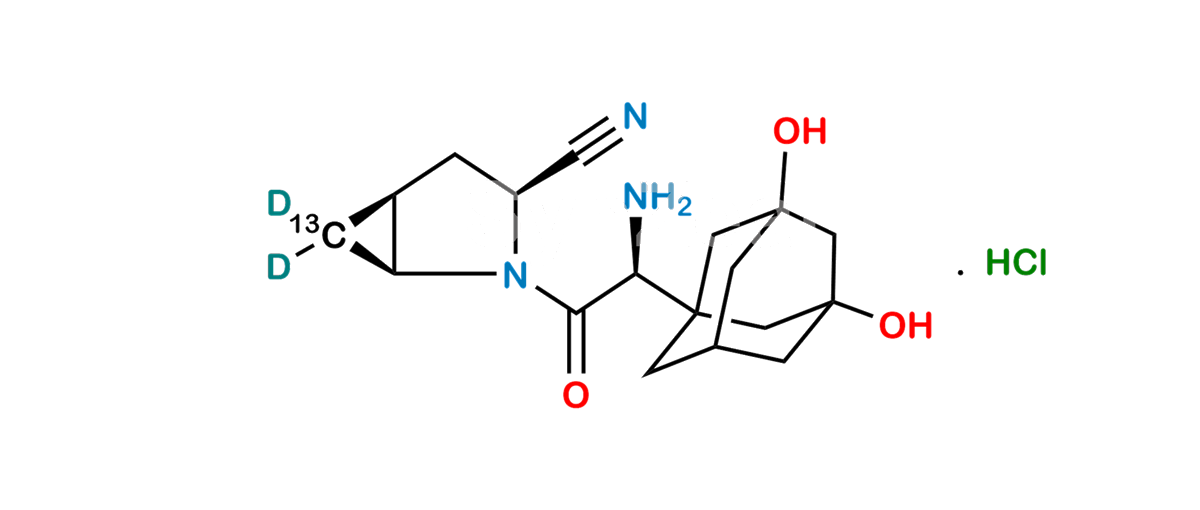
Chemical Name : (1S,3S,5S)-2-[(2S)-2-Amino-2-(3,5-dihydroxytricyclo[3.3.1.13,7]dec-1-yl)acetyl]-2-Azabicyclo[3.1.0]hexane-3-carbonitrile-13C,D2 Hydrochloride;
M2 Saxagliptin Hydroxylated Metabolite-13C,D2 Hydrochloride
Smiles : N[C@@H]([C@](C[C@@](C1)(O)C2)(C[C@@H]1C3)C[C@]23O)C(N([C@@H](C4)C#N)[C@@H]5[C@H]4[13C]5([2H])[2H])=O.Cl
Inchi : InChI=1S/C18H25N3O3.ClH/c19-6-12-1-11-2-13(11)21(12)15(22)14(20)16-3-10-4-17(23,7-16)9-18(24,5-10)8-16;/h10-14,23-24H,1-5,7-9,20H2;1H/t10-,11-,12+,13+,14-,16-,17+,18-;/m1./s1/i2D2,19+1;
Technical Data
Reference
Development and validation of RP-HPLC method for simultaneous estimation of saxagliptin and dapagliflozin
By Desai, Charmy P.; Chaudhary, Ankit B.; Patel, Bhoomi D.nFrom World Journal of Pharmacy and Pharmaceutical Sciences (2018), 7(5), 803-812
Gradient elution RP-HPLC method for the determination of related substances in saxagliptin
By Li, Xiaoyan; Liu, Fei – From Zhongguo Yaopin Biaozhun (2015), 16(1), 16-19
Development and validation of simple stability indicating RP-HPLC method for analysis of saxagliptin and its forced degradation impurities in bulk drug and pharmaceutical dosage form
By Chhabda, Pawanjeet J.; Balaji, M.; Srinivasarao, V.; Ramakrishna, K.; Apparao, K. M. Ch. – From International Journal of Research and Development in Pharmacy & Life Sciences (2014), 3(3), 993-1003, 11 pp..
RFQ