No products in the cart.
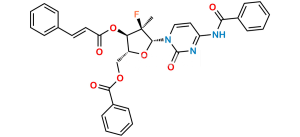
Sofosbuvir Impurity 28

Product Description
CAT No.
ALN-S010051
CAS No.
NA
Mol. F.
C20H19ClO6
Mol. Wt.
390.8
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
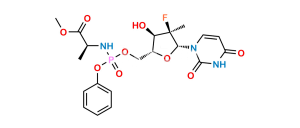
Chemical Name : ((2R,3R,4S)-3-(Benzoyloxy)-5-chloro-4-hydroxy-4-methyltetrahydrofuran-2-yl)methyl benzoate
Smiles : O=C(C1=CC=CC=C1)OC[C@H]2OC(Cl)[C@@](C)(O)[C@@H]2OC(C3=CC=CC=C3)=O
Inchi : InChI=1S/C20H20O7/c1-20(24)16(27-18(22)14-10-6-3-7-11-14)15(26-19(20)23)12-25-17(21)13-8-4-2-5-9-13/h2-11,15-16,19,23-24H,12H2,1H3/t15-,16-,19?,20+/m1/s1
Technical Data
Reference
Method development and validation for the simultaneous estimation of sofosbuvir and velpatasvir in bulk and tablet dosage forms by RP-HPLC
By Suma, M. S. M.; Gowthami, M.; Latha, P. V. Madhavi; Devi, P. UmanFrom Journal of Global Trends in Pharmaceutical Sciences (2021), 12(1), 9037-9043.
Development and validation of RP-HPLC method for the estimation of Sofosbuvir and Ledipasvir in combined dosage form
By Prabhaker, Naik Desai Prabhat; Pankaj, Kapupara – From International Journal of Research in Pharmaceutical Sciences (Madurai, India) (2021), 12(1), 523-529.
Eco-friendly HPTLC method for simultaneous analysis of sofosbuvir and ledipasvir in biological and pharmaceutical samples: Stability indicating study
By El-Yazbi, Amira F.; Elashkar, Nourhan E.; Abdel-Hay, Karim M.; Talaat, Wael; Ahmed, Hytham M. – From Microchemical Journal (2020), 154, 104584.
RFQ