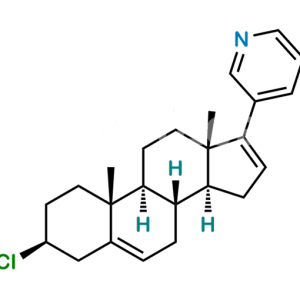
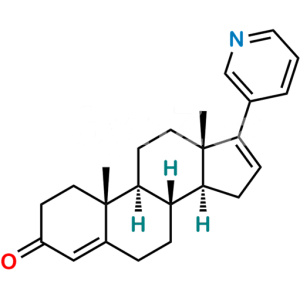
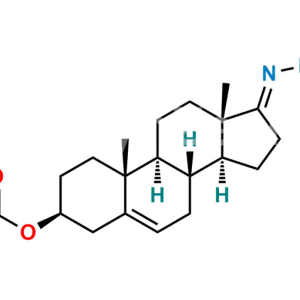
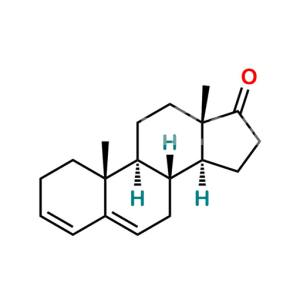
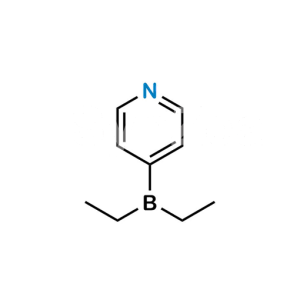
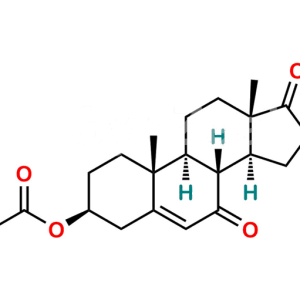
Dehydroandrosterone Acetate

Product Description
CAT No.
ALN-A006049
CAS No.
5223-99-4
Mol. F.
C21H30O3
Mol. Wt.
330.5
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
Chemical Name : (3R,8R,9S,10R,13S,14S)-10,13-dimethyl-17-oxo-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl acetate
Smiles : C[C@@](C1=CC2)(CC[C@@H](OC(C)=O)C1)[C@]3([H])[C@]2([H])[C@@](CCC4=O)([H])[C@]4(C)CC3
Inchi : InChI=1/C16H15F2N3O4S/c1-23-13-5-6-19-12(14(13)24-2)8-26(22)16-20-10-4-3-9(25-15(17)18)7-11(10)21-16/h3-7,15H,8H2,1-2H3,(H,20,21)/i1D3,2D3,5D,6D
Synonym : 3α-Acetoxyandrost-5-en-17-one
Technical Data
Reference
Identification, Characterization and HighPerformance Liquid Chromatography Quantification for Process-Related Impurities in Abiraterone Acetate Bulk Drug
Changjie Hu, Hanqiao Zhang, Menglin Zhang, Zhiyuan Mi, Jun Wang, Wenpin Lu, and Jiangtao Su*nJournal of Chromatographic Science, Volume 56, Issue 9, October 2018, Pages 802u2013811
stability indicating rp-hplc-pda method for determination of abiraterone acetate and characterization of its base catalyzed degradation product by lc-ms
– Journal of Chromatographic Science, Volume 56, Issue 9, October 2018, Pages 802–811
Department of Quality Assurance Techniques, maeer’S Maharashtra Institute of Pharmacy, MIT Campus, Kothrud, Pune 411038 – International Journal of Pharmacy and Pharmaceutical Sciences, Vol 8, Issue 2, 2016
RFQ