No products in the cart.
Acarbose EP Impurity G

Product Description
CAT No.
ALN-A091008
CAS No.
1013621-73-2
Mol. F.
C31H53NO23
Mol. Wt.
807.7
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
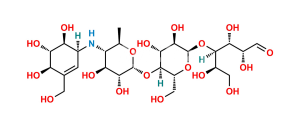
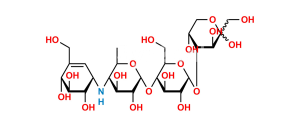
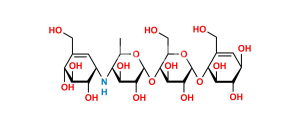
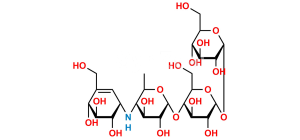
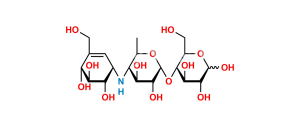
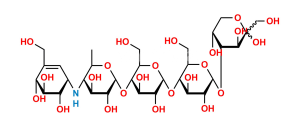
Chemical Name : α-D-Glucopyranosyl O-4,6-dideoxy-4-[[(1S,4R,5S,6S)-4,5,6-trihydroxy-3-(hydroxymethyl)cyclohex-2-enyl]amino]-α-D-glucopyranosyl-(1→4)-O-α-D-glucopyranosyl-(1→4)-O-α-D-glucopyranoside (as per EP & USP)
Smiles : C[C@H]1O[C@H](O[C@H]2[C@H](O)[C@@H](O)[C@@H](O[C@H]3[C@H](O)[C@@H](O)[C@@H](O[C@H]4O[C@H](CO)[C@@H](O)[C@H](O)[C@H]4O)O[C@@H]3CO)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@@H]1N[C@@H]5[C@H](O)[C@@H](O)[C@H](O)C(CO)=C5
Inchi : InChI=1S/C31H53NO23/c1-7-13(32-9-2-8(3-33)14(37)17(40)15(9)38)16(39)22(45)29(49-7)53-26-11(5-35)51-31(23(46)19(26)42)55-27-12(6-36)52-30(24(47)20(27)43)54-25-10(4-34)50-28(48)21(44)18(25)41/h2,7,9-48H,3-6H2,1H3/t7-,9+,10-,11-,12-,13-,14-,15+,16+,17+,18-,19-,20-,21-,22-,23-,24-,25-,26-,27-,28?,29-,30-,31-/m1/s1
Synonym : Acarbose USP impurity G
Technical Data
Reference
Isolation and preparation methods of acarbose impurities
By Liang, Xianrui; Zhang, Huichen; He, Xiaoai; Wu, Hui; Su, WeikenFrom Zhejiang Gongye Daxue Xuebao (2017), 45(3), 289-293
An innovative method for estimation of metformin HCl and acarbose pharmaceutical products and separation of metformin impurities by RP-HPLC
By Shaikh, Jalil K.; M., Ajay Babu; Farooqui, Mazahar; Syed, Ummul Khair Asema – From International Journal of PharmTech Research (2020), 13(2), 71-79
Development and Validation of a Stability-Indicating HPLC Method for the Determination of Acarbose in Pharmaceutical Dosage Forms
By Azam Sadat Montazeri; Mohammadi, Ali; Adib, Noushin; Naeemy, Ali – From Journal of Analytical Chemistry (2018), 73(9), 910-916
RFQ