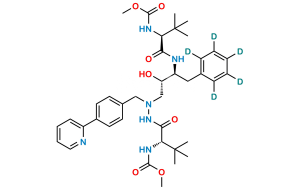
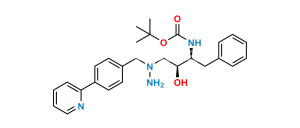
Atazanavir Impurity 7

Product Description
CAT No.
ALN-A009026
CAS No.
1192224-26-2
Mol. F.
C36H50N6O5
Mol. Wt.
646.8
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
Chemical Name : Methyl ((S)-1-(2-((2S,3S)-3-((S)-2-amino-3,3-dimethylbutanamido)-2-hydroxy-4-phenylbutyl)-2-(4-(pyridin-2-yl)benzyl)hydrazinyl)-3,3-dimethyl-1-oxobutan-2-yl)carbamate
Smiles : O=C([C@@H](N)C(C)(C)C)N[C@@H](CC1=CC=CC=C1)[C@@H](O)CN(CC2=CC=C(C3=NC=CC=C3)C=C2)NC([C@H](C(C)(C)C)NC(OC)=O)=O
Inchi : InChI=1S/C32H42N4O5/c1-31(2,3)40-29(38)34-27(20-23-12-8-7-9-13-23)28(37)22-36(35-30(39)41-32(4,5)6)21-24-15-17-25(18-16-24)26-14-10-11-19-33-26/h7-19,27-28,37H,20-22H2,1-6H3,(H,34,38)(H,35,39)/t27-,28+/m0/s1
Technical Data
Reference
Determination of Genotoxic impurity in Atazanavir sulphate drug substance by LC-MS
K. Geetha Bhavania , K. Bala Murali Krishnac , N. Srinivasub* , D. Ramachandranc* , N.V.V.S.S. Ramand , B. Hari Babuc*nJ Pharm Biomed Anal. 2017 Jan 5;132:156-158
Impact of monomeric vs. micellar surfactant and surfactant-polymer interactions on nucleation-induction times of atazanavir from supersaturated solutions
, D. Ramachandranc
Simultaneous Determination of Impurities of Atazanavir and Ritonavir in Tablet Dosage Form by Using Reversed-Phase Ultra Performance Liquid Chromatographic Method
, N.V.V.S.S. Ramand , B. Hari Babuc
RFQ