No products in the cart.
Atenolol EP Impurity F

Product Description
CAT No.
ALN-A035007
CAS No.
87619-83-8
Mol. F.
C25H35N3O6
Mol. Wt.
473.6
Stock
In Stock
Product Overview
Technical Data
Reference
RFQ
Product Overview
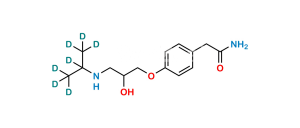
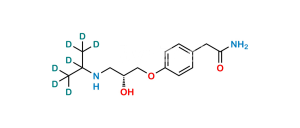
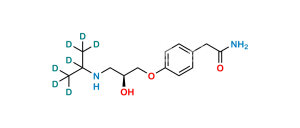
Chemical Name : 2,2′-[(1-Methylethyl)iminobis(2-hydroxypropan-3,1-diyloxy-4,1-phenylene)]diacetamide
Smiles : CC(N(CC(O)COC1=CC=C(CC(N)=O)C=C1)CC(O)COC2=CC=C(CC(N)=O)C=C2)C
Inchi : InChI=1S/C19H22N2O5/c20-18(23)9-13-1-5-16(6-2-13)25-11-15(22)12-26-17-7-3-14(4-8-17)10-19(21)24/h1-8,15,22H,9-12H2,(H2,20,23)(H2,21,24)
Synonym : Atenolol USP Related Compound F
Technical Data
Reference
The assay and resolution of the beta-blocker atenolol from its related impurities in a tablet pharmaceutical dosage form
Zen pawlak* and Brian j. clarknJournal of Pharmaceutical & Biomedical Analysis Vol. 10, No. 5, pp. 329-334,1992
Removal of Atenolol and Isoproturon in Aqueous Solutions by Adsorption in a Fixed-Bed Column
and Brian j. clark – Journal of Pharmaceutical & Biomedical Analysis Vol. 10, No. 5, pp. 329-334,1992
A simple, selective, and sensitive gas chromatography-mass spectrometry method for the analysis of five process-related impurities in atenolol bulk drug and capsule formulations
JoséLuis Sotelo, Gabriel Ovejero, Araceli Rodríguez, Silvia Á lvarez, and Juan García – Ind. Eng. Chem. Res. 2012, 51, 5045−5055
RFQ