No products in the cart.
Betamethasone Valerate EP Impurity H
Product Description
CAT No.
ALN-B025036
CAS No.
52619-18-8
Mol. F.
C27H37ClO6
Mol. Wt.
493
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
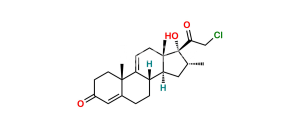
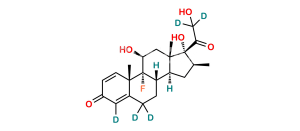
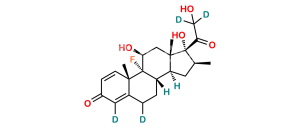
Chemical Name : 9-Chloro-11β,21-dihydroxy-16β-methyl-3,20-dioxopregna-1,4-dien-17-yl pentanoate ;
Smiles : CCCCC(O[C@]1(C(CO)=O)[C@@H](C)C[C@@]2([H])[C@]3([H])CCC4=CC(C=C[C@]4(C)[C@@]3(Cl)[C@@H](O)C[C@]12C)=O)=O
Inchi : InChI=1S/C27H36BrFO6/c1-5-6-7-23(34)35-27(22(33)14-30)15(2)10-17-18-12-20(28)19-11-16(31)8-9-24(19,3)26(18,29)21(32)13-25(17,27)4/h8-9,11,15,17-18,20-21,30,32H,5-7,10,12-14H2,1-4H3/t15-,17-,18-,20-,21-,24-,25-,26-,27-/m0/s1
Synonym : Beclomethasone 17-Valerate ;
Technical Data
Reference
Application of LCu2013MSn in conjunction with mechanism-based stress studies in the elucidation of drug impurity structure: Rapid identification of a process impurity in betamethasone 17-valerate drug substance
Min Li, Mingxiang Lin, Abu RustumnJournal of Pharmaceutical and Biomedical Analysis 48 (2008) 1451u20131456
Development and Validation of Stability-indicating HPLC Method for Betamethoasone Dipropionate and Related Substances in Topical Formulation
A. s. vairale
, p. sivaswaroop1 and s. bandana – Indian Journal of Pharmaceutical Sciences 74(2):107-15
RFQ