No products in the cart.
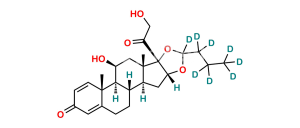
Budesonide D6

Product Description
CAT No.
ALN-B022D02
CAS No.
1134189-63-1
Mol. F.
C25H28D6O6
Mol. Wt.
436.6
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
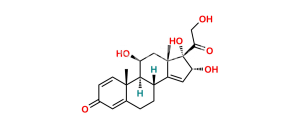
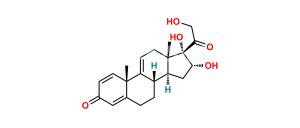
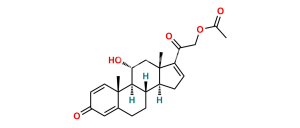
Chemical Name : (6aR,6bS,7S,8aS,8bS,11aR,12aS,12bS)-7-Hydroxy-8b-(2-hydroxyacetyl-d2)-6a,8a-dimethyl-10-propyl-1,2,6a,6b,7,8,8a,8b,11a,12,12a,12b-dodecahydro-4H-naphtho[2′,1′:4,5]indeno[1,2-d][1,3]dioxol-4-one-2,2,3,5-d4
Smiles : O=C([C@]([C@@]1([H])C[C@@]2([H])[C@@](CC([2H])([2H])C3=C(C4=O)[2H])([H])[C@]([C@]3(C=C4[2H])C)([H])[C@@H](O)C5)(OC(CCC)O1)[C@]25C)C([2H])([2H])O
Inchi : InChI=1S/C5H12N2.H/c6-5-2-1-3-7-4-5;/h5,7H,1-4,6H2;/t5-;/m0./s1/i;1+1
Technical Data
Reference
A validated stability indicating UHPLC method for the determination of anti-inflammatory corticosteroid budesonide epimers
By Vaddi, Lakshmi; Purushothaman, Vijayanthimala; Gowda, NagarajnFrom Journal of Pharmaceutical Sciences and Research (2020), 12(6), 848-852
Analytical method development and validation of formoterol fumarate and budesonide in pressurized meter dose inhaler form by using RP-HPLC
By Raghu, P. S. – From European Journal of Biomedical and Pharmaceutical Sciences (2018), 5(6), 1-6
Isolation and characterization of photodegradation impurity in budesonide drug product using LC-MS and NMR spectroscopy
By Bhutnar, Arun; Khapare, Sachin; Desai, Anita; Dsouza, Smitha – From American Journal of Analytical Chemistry (2017), 8(7), 449-461
RFQ