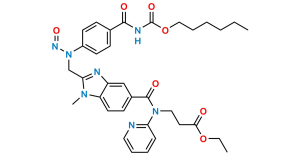
Dabigatran Impurity 14

Product Description
CAT No.
ALN-D012037
CAS No.
NA
Mol. F.
C40H55N7O7
Mol. Wt.
745.9
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
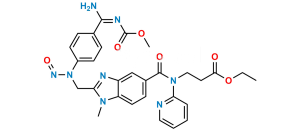
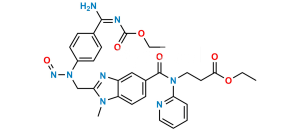
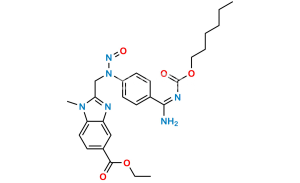
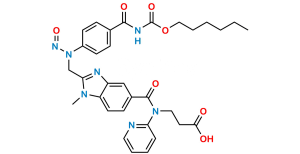
Chemical Name : Methyl 3-(4-(((hexyloxy)carbonyl)amino)-3-((2-((4-(N-((hexyloxy)carbonyl)carbamimidoyl)phenyl)amino)ethyl)(methyl)amino)-N-(pyridin-2-yl)benzamido)propanoate
Smiles : CCCCCCOC(NC(C1=CC=C(NCCN(C)C2=CC(C(N(CCC(OC)=O)C3=CC=CC=N3)=O)=CC=C2NC(OCCCCCC)=O)C=C1)=N)=O
Inchi : InChI=1S/C55H53N13O7.CH4O3S/c1-5-74-49(69)25-29-67(45-11-7-9-27-56-45)53(71)37-17-23-43-41(31-37)60-47(65(43)3)33-58-39-19-13-35(14-20-39)51-62-52(64-55(73)63-51)36-15-21-40(22-16-36)59-34-48-61-42-32-38(18-24-44(42)66(48)4)54(72)68(30-26-50(70)75-6-2)46-12-8-10-28-57-46;1-5(2,3)4/h7-24,27-28,31-32,58-59H,5-6,25-26,29-30,33-34H2,1-4H3,(H,62,63,64,73);1H3,(H,2,3,4)
Technical Data
Reference
Novel method for separation and quantification of potential impurities by RP-HPLC from KSM stage to API stage of dabigatran mesylate
By Jhansi, T. Naga; Nagaraju, R.; Kumar, D. Jaya Deep; Rao, G. NageswaranFrom International Journal of Pharmaceutical Sciences and Research (2021), 12(3), 1762-1779
Development and Validation of RP-UPLC Method for the Determination of Process and Degradant Impurities Present in Dabigatran Etexilate Mesylate Capsules Using High Strength Silica-T3 Sorbent Column
By Mantena, Bhaskara P. V.; Rao, Sumathi V.; Suryakala, D.; Ramakrishna, K.; Srikanth Reddy, R. – From Analytical Chemistry Letters (2016), 6(5), 595-611
Determination of dabigatran etexilate mesylate by HPLC
By Jia, Ling-xiao; Tang, Lei; Zhang, Shu-ting; Song, Rui-zhi; Gao, Zhan-wen – From Shipin Yu Yaopin (2015), 17(3), 195-197
RFQ