No products in the cart.
Daclatasvir SRRR Isomer

Product Description
CAT No.
ALN-D036013
CAS No.
NA
Mol. F.
C40H50N8O6
Mol. Wt.
738.9
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
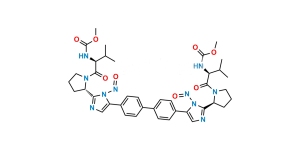
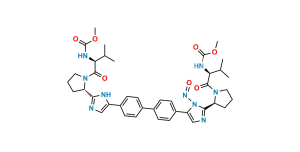
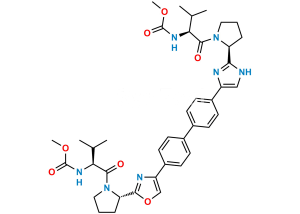
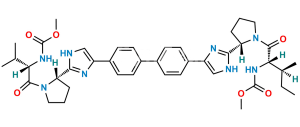
Chemical Name : Dimethyl (2S,2’R)-1,1′-((2R,2’R)-2,2′-(5,5′-(biphenyl-4,4′-diyl)bis(1H-imidazole-5,2-diyl))bis(pyrrolidine-2,1-diyl))bis(3-methyl-1-oxobutane-2,1-diyl)dicarbamate
Smiles : O=C([C@@H](NC(OC)=O)C(C)C)N1CCC[C@@H]1C2=NC=C(N2)C3=CC=C(C4=CC=C(C5=CN=C([C@H]6CCCN6C([C@H](NC(OC)=O)C(C)C)=O)N5)C=C4)C=C3
Inchi : InChI=1S/C20H25BrN4O3/c1-12(2)17(24-20(27)28-3)19(26)25-10-4-5-16(25)18-22-11-15(23-18)13-6-8-14(21)9-7-13/h6-9,11-12,16-17H,4-5,10H2,1-3H3,(H,22,23)(H,24,27)/t16-,17+/m1/s1
Technical Data
Reference
A validated stability-indicating reverse-phase high-performance liquid chromatography method for daclatasvir, identification and characterization of degradation products using LC-ESI-QTOF-MS
By Warghade, Snehal V.; Bothara, Kailash G.nFrom Asian Journal of Pharmaceutical and Clinical Research (2019), 12(5), 302-308
Development and validation of HPLC fluorescence and UPLC/DAD stability-indicating methods for determination of hepatitis C antiviral agent daclatasvir
By Kamal, Andra H.; Ismail, Nahla S.; Mabroijk, Mokhtar M.; Bebawy, Lories I.; Mekky, Mai A. – From Journal of AOAC International (2019), 102(4), 1125-1131
A stability-indicating UPLC method for the determination of potential impurities and its mass by a new QDa mass detector in daclatasvir drug used to treat hepatitis C infection
By Jagadabi, Varaprasad; Kumar, P. V. Nagendra; Mahesh, Kasthuri; Pamidi, Srinivasu; Ramaprasad, L. A.; Nagaraju, D. – From Journal of Chromatographic Science (2019), 57(1), 44-53
RFQ