No products in the cart.
Equilenin Impurity 3

Product Description
CAT No.
ALN-E036005
CAS No.
NA
Mol. F.
C22H32O2
Mol. Wt.
328.5
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
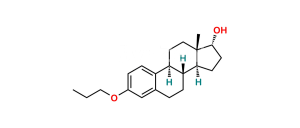
Chemical Name : (8R,9S,13S,14S,17R)-17-Methoxy-13-methyl-3-propoxy-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene
Smiles : C[C@@]1([C@@H]2OC)[C@](CC2)([H])[C@@](CCC3=CC(OCCC)=CC=C43)([H])[C@]4([H])CC1
Inchi : InChI=1S/C21H30O2/c1-3-12-23-15-5-7-16-14(13-15)4-6-18-17(16)10-11-21(2)19(18)8-9-20(21)22/h5,7,13,17-20,22H,3-4,6,8-12H2,1-2H3/t17-,18-,19+,20-,21+/m1/s1
Technical Data
Reference
Formation of equilenin from equilin by porcine adrenals
K. L. Cheo and K. H. LokenSteroids Volume 11, Issue 5, May 1968, Pages 603-608
The Major Metabolite of Equilin, 4-Hydroxyequilin, Autoxidizes to an o-Quinone Which Isomerizes to the Potent Cytotoxin 4-Hydroxyequilenin-o-quinone
Fagen Zhang, Yumei Chen, Emily Pisha, Li Shen, Yansan Xiong, Richard B. van Breemen, and Judy L. Bolton – Chem. Res. Toxicol. 1999, 12, 204-213
RFQ