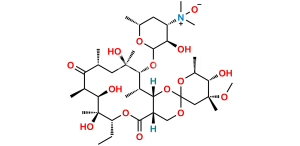
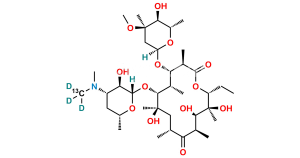
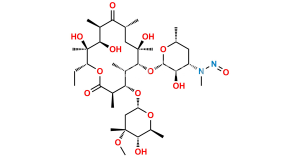
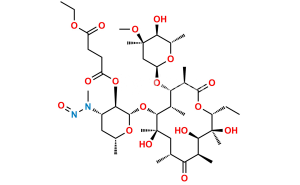
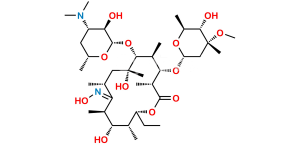
Erythromycin Sulfate

Product Description
CAT No.
ALN-E006021
CAS No.
7184-72-7
Mol. F.
C37H67NO13 : H2SO4
Mol. Wt.
733.9 : 981
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
Chemical Name : (3R,4S,5S,6R,7R,9R,11R,12R,13S,14R)-4-[(2,6-Dideoxy-3-C-methyl-3-O-methyl-α-L-ribo-hexopyranosyl)oxy]-14-ethyl-7,12,13-trihydroxy-3,5,7,9,11,13-hexamethyl-6-[[3,4,6-trideoxy-3-(dimethylamino)-β-D-xylo-hexopyranosyl]oxy]-1-oxacyclotetradecane-2,10-dione Sulfate (as per EP);
(3R*,4S*,5S*,6R*,7R*,9R*,11R*,12R*,13S*,14R*)-4-[(2,6-Dideoxy-3-C-methyl-3-O-methyl-α-Lribohexopyranosyl)oxy]-14-ethyl-7,12,13-trihydroxy-3,5,7,9,11,13-hexamethyl-6-[[3,4,6-trideoxy-3-
(dimethylamino)-β-D-xylo-hexopyranosyl]oxy]oxacyclotetradecane-2,10-dione Sulfate (as per USP)
Smiles : C[C@@H]([C@@H]([C@H](C(O[C@@H]1CC)=O)C)O[C@@](O[C@@H](C)[C@@H]2O)([H])C[C@@]2(C)OC)[C@H]([C@](O)(C[C@H](C([C@@H]([C@@H](O)[C@@]1(O)C)C)=O)C)C)O[C@@](O[C@H](C)C[C@@H]3N(C)C)([H])[C@@H]3O.O=[S](O)(O)=O
Inchi : InChI=1S/C37H67NO13.ClH/c1-14-25-37(10,45)30(41)20(4)27(39)18(2)16-35(8,44)32(51-34-28(40)24(38(11)12)15-19(3)47-34)21(5)29(22(6)33(43)49-25)50-26-17-36(9,46-13)31(42)23(7)48-26;/h18-26,28-32,34,40-42,44-45H,14-17H2,1-13H3;1H/t18-,19-,20+,21+,22-,23+,24+,25-,26+,28-,29+,30-,31+,32-,34+,35-,36-,37-;/m1./s1
Technical Data
Reference
Isolation and identification of a novel erythromycin-degrading fungus, Curvularia sp. RJJ-5, and its degradation pathway
By Ren, Jianjun; Deng, Liujie; Niu, Dongze; Wang, Zhenzhu; Fan, Bo; Taoli, Huhe; Li, Zhijie; Zhang, Jin; Li, ChunyunFrom FEMS Microbiology Letters (2021), 368(1), fnaa215.
Development and validation of a stability indicating HPLC method for organic impurities of erythromycin stearate tablets
By Jeelani, Salika; Soukhova, Nadejda – From Journal of Pharmaceutical and Biomedical Analysis (2021), 195, 113858
Degradation and mineralization of erythromycin by heterogeneous photocatalysis using SnO2-doped TiO2 structured catalysts: Activity and stability
By Albornoz, L. L.; da Silva, S. W.; Bortolozzi, J. P.; Banus, E. D.; Brussino, P.; Ulla, M. A.; Bernardes, A. M. – From Chemosphere (2021), 268, 128858
RFQ