No products in the cart.
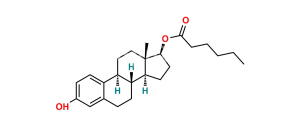
Estradiol Valerate EP Impurity I

Product Description
CAT No.
ALN-E005010
CAS No.
NA
Mol. F.
C49H66O5
Mol. Wt.
735.1
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
Chemical Name : (1S,3aS,3bR,10aR,10bS,13S,13aS,15aS,18bS,20aS)-13a,17,17,20a-tetramethyl-2,3,3a,3b,4,5,9,10,10a,10b,11,12,13,13a,14,15,15a,17,18b,19,20,20a-docosahydro-1H-bis(cyclopenta[5,6]naphtho)[1,2-b:2ʹ,1ʹ-i]xanthene-1,13-diyl dipentanoate (as per EP)
Smiles : C[C@@]12[C@](OC(CCCC)=O)([H])CC[C@@]1([H])[C@]3([H])CCC4=C(C=C(C(C)(C)C(C=C([C@@](CC[C@]5(C)[C@]6([H])CC[C@@]5([H])OC(CCCC)=O)([H])[C@]6([H])CC7)C7=C8)=C8O9)C9=C4)[C@@]3([H])CC2
Inchi : InChI=1S/C46H55F6N3O4/c1-41-19-16-30-26(27(41)9-11-32(41)39(58)53-34-23-24(45(47,48)49)5-8-31(34)46(50,51)52)7-14-36-44(30,4)22-18-38(57)55(36)40(59)33-12-10-28-25-6-13-35-43(3,21-17-37(56)54-35)29(25)15-20-42(28,33)2/h5,8,17-18,21-23,25-30,32-33,35-36H,6-7,9-16,19-20H2,1-4H3,(H,53,58)(H,54,56)/t25-,26-,27-,28-,29-,30-,32+,33-,35+,36+,41-,42-,43+,44+/m0/s1
Technical Data
Reference
Validated RP-HPLC method for the determination of estradiol valerate in bulk and pharmaceutical formulations
M. Madhu1 *, Satyadev T. N. V. S. S.1 , G. Hephzibah1 and T. V. ReddynDer Pharmacia Lettre, 2016, 8 (4):50-61
Determination of estradiol and its degradation products by liquid chromatography
, Satyadev T. N. V. S. S.1 , G. Hephzibah1 and T. V. Reddy – Der Pharmacia Lettre, 2016, 8 (4):50-61
Determination of Related Substances in Estradiol Valerate Tablet by Use of HPLC Method
Lucie Havlíková, Lucie Nováková, Ludmila Matysová, Jan Sícha – Journal of Chromatography A 1119(1-2):216-23
RFQ