Etodolac EP Impurity C
Product Description
CAT No.
ALN-E023004
CAS No.
109518-47-0
Mol. F.
C16H19NO3
Mol. Wt.
273.3
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
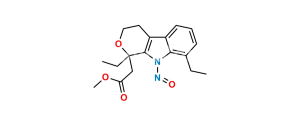
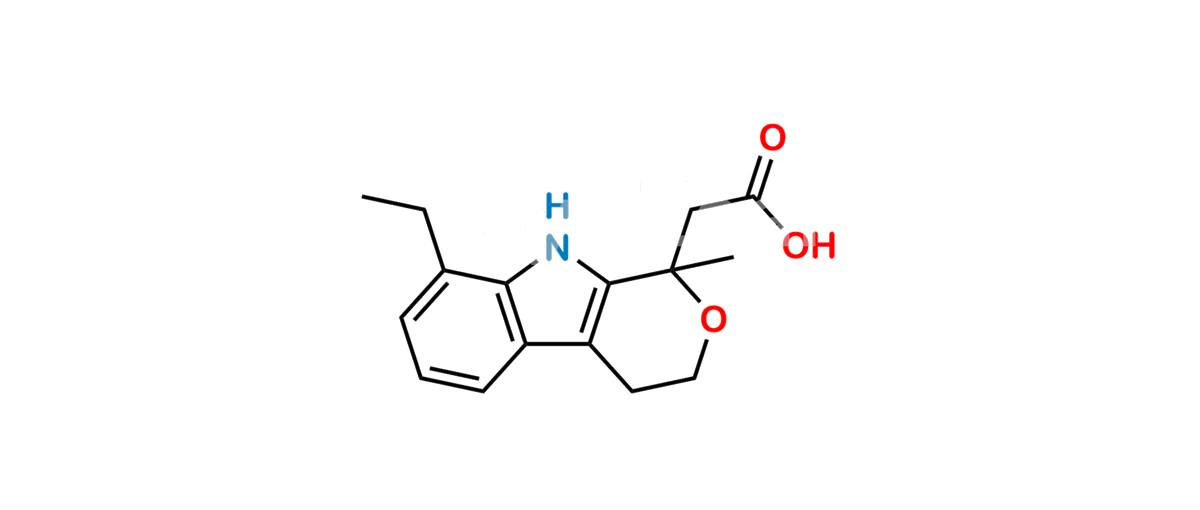
Chemical Name : (1RS)-8-ethyl-1-methyl-1,3,4,9-tetrahydropyrano[3,4-b]indol-1-yl]acetic acid (as per EP);
(+/-)-8-Ethyl-1-methyl-1,3,4,9-tetrahydropyrano [3,4-b]-indole-1-acetic acid (as per USP)
Smiles : O=C(O)CC1(C)OCCC2=C1NC3=C2C=CC=C3CC
Inchi : InChI=1/C16H19NO3/c1-3-16(9-13(18)19)15-12(7-8-20-16)11-6-4-5-10(2)14(11)17-15/h4-6,17H,3,7-9H2,1-2H3,(H,18,19)
Synonym : Etodolac USP Related Compound A ; Entecavir USP Impurity I ; 1-Methyl Etodolac
Technical Data
Reference
Method development and validation of HPLC for simultaneous determination of etodolac
By Srinivasarao, Kandimalla; Pai, K. Vasantha KumarnFrom Pharma Chemica (2015), 7(3), 284-288
Improvement of the etodolac purity test by reversed phase high-performance liquid chromatography
By Ammar, A.; Surmann, P. – From Pharmazie (2008), 63(12), 913-914
Determination of enantiomeric impurity of etodolac by capillary electrophoresis using (2-hydroxypropyl)-β-cyclodextrin
By Dung, Phan Thanh; Ko, Mi Young; Choi, Hyun Ju; Sin, Kwan Seog; Kim, Kyeong Ho – From Archives of Pharmacal Research (2008), 31(9), 1218-1223
RFQ