No products in the cart.
Exemestane EP Impurity D

Product Description
CAT No.
ALN-E025004
CAS No.
897-06-3
Mol. F.
C19H24O2
Mol. Wt.
284.4
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
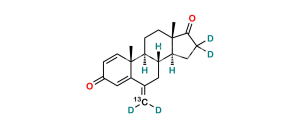
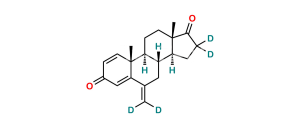
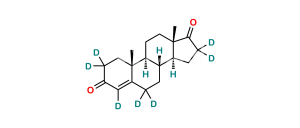
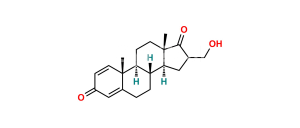
Chemical Name : Androsta-1,4-diene-3,17-dione (as per EP & USP)
Smiles : C[C@]1([C@](CC2)([H])[C@]3([H])CCC4=CC(C=C[C@]4(C)[C@@]3([H])CC1)=O)C2=O
Inchi : InChI=1S/C21H24O2/c1-12-9-15-16(20(3)7-5-14(22)11-17(12)20)6-8-21(4)18(15)10-13(2)19(21)23/h5,7,11,15-16,18H,1-2,6,8-10H2,3-4H3/t15-,16+,18+,20-,21+/m1/s1
Synonym : Exemestane USP Related Compound C ; 1,4-Androstadiendione ; 1-Dehydroandrostenedione ; Androstadienedione
Technical Data
Reference
Development of validated method for the determination of exemestane by using RP-HPLC
By Siva Sai Kiran, B.; Raja, S.nFrom International Journal of Research in Pharmaceutical Sciences (Madurai, India) (2018), 9(1), 185-188
Use of the hyphenated LC-MS/MS technique and NMR/IR spectroscopy for the identification of exemestane stress degradation products during the drug development
By Stolarczyk, Elzbieta U.; Rosa, Anna; Kubiszewski, Marek; Zagrodzka, Joanna; Cybulski, Marcin; Kaczmarek, Lukasz – From European Journal of Pharmaceutical Sciences (2017), 109(Suppl.), 389-401
A validated stability-indicating liquid chromatographic method for the determination of Exemestane
By Mathrusri Annapurna, M.; Swati, B.; Naga Ramya, D.; Pramadvara, K.; Sri Ram, A.; Aslesha, N. – From Chemical Science Transactions (2014), 3(3), 961-968, 8 pp..
RFQ