Ezetimibe D4-O-Beta-Glucuronide

Product Description
CAT No.
ALN-E026D04
CAS No.
NA
Mol. F.
C30H25D4F2NO9
Mol. Wt.
589.6
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
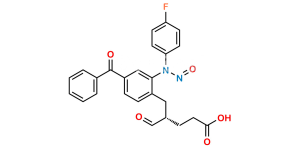
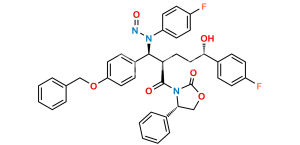
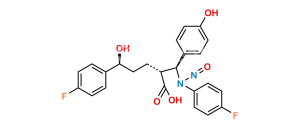
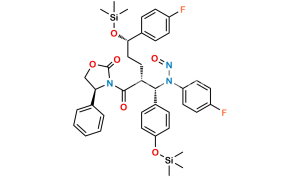
Chemical Name : (2S,3S,4S,5R,6S)-6-(4-((2S,3R)-1-(4-fluorophenyl)-3-((S)-3-(4-fluorophenyl-2,3,5,6-d4)-3-hydroxypropyl)-4-oxoazetidin-2-yl)phenoxy)-3,4,5-trihydroxytetrahydro-2H-pyran-2-carboxylic acid
Smiles : O[C@@H](CC[C@@H]1[C@H](N(C1=O)C2=CC=C(F)C=C2)C3=CC=C(C=C3)O[C@H](O[C@@H]4C(O)=O)[C@H](O)[C@H]([C@@H]4O)O)C5=C([2H])C([2H])=C(C([2H])=C5[2H])F
Technical Data
Reference
Related substances by HPLC method for the detection and evaluation of impurities in ezetimibe drug material
By Rapeti, Durgababu; Reddy, Gudibanda Chandra Sekhar; Narayanarao, Kapavarapu Maruthi Venkata; Shyamala, Pulipaka; Krishna, Rallabhandi MuralinFrom International Journal of Pharmaceutical Sciences and Research (2021), 12(1), 217-225
Stability-indicating liquid chromatographic method for the simultaneous determination of rosuvastatin and ezetimibe from pharmaceuticals and biological samples
By Kurbanoglu, Sevinc; Esim, Ozgur; Ozkan, Cansel Kose; Savaser, Ayhan; Ozkan, Yalcin; Uslu, Bengi; Ozkan, Sibel A. – From Journal of the Turkish Chemical Society, Section A: Chemistry (2020), 7(3), 865-874
Stability-indicating analytical method development using quality by design approach for simultaneous estimation of ezetimibe and glimepiride
By Shah, U.; Shah, Kunti; Patel, Rupal – From Indian Journal of Pharmaceutical Sciences (2019), 81(2), 273-281
RFQ