No products in the cart.
Ezetimibe Impurity 4

Product Description
CAT No.
ALN-E026054
CAS No.
189028-93-1
Mol. F.
C20H18FNO4
Mol. Wt.
355.4
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
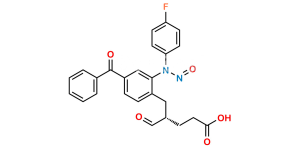
Chemical Name : (S)-1-(4-Fluorophenyl)-5-(2-oxo-4-phenyloxazolidin-3-yl)pentane-1,5-dione
Smiles : O=C(N1[C@@H](C2=CC=CC=C2)COC1=O)CCCC(C3=CC=C(F)C=C3)=O
Inchi : InChI=1S/C20H20FNO4/c21-16-11-9-15(10-12-16)18(23)7-4-8-19(24)22-17(13-26-20(22)25)14-5-2-1-3-6-14/h1-3,5-6,9-12,17-18,23H,4,7-8,13H2/t17-,18+/m0/s1
Technical Data
Reference
Related substances by HPLC method for the detection and evaluation of impurities in ezetimibe drug material
By Rapeti, Durgababu; Reddy, Gudibanda Chandra Sekhar; Narayanarao, Kapavarapu Maruthi Venkata; Shyamala, Pulipaka; Krishna, Rallabhandi MuralinFrom International Journal of Pharmaceutical Sciences and Research (2021), 12(1), 217-225
Stability-indicating liquid chromatographic method for the simultaneous determination of rosuvastatin and ezetimibe from pharmaceuticals and biological samples
By Kurbanoglu, Sevinc; Esim, Ozgur; Ozkan, Cansel Kose; Savaser, Ayhan; Ozkan, Yalcin; Uslu, Bengi; Ozkan, Sibel A. – From Journal of the Turkish Chemical Society, Section A: Chemistry (2020), 7(3), 865-874
Stability-indicating analytical method development using quality by design approach for simultaneous estimation of ezetimibe and glimepiride
By Shah, U.; Shah, Kunti; Patel, Rupal – From Indian Journal of Pharmaceutical Sciences (2019), 81(2), 273-281
RFQ