No products in the cart.
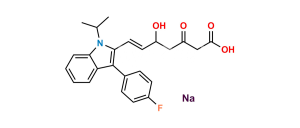
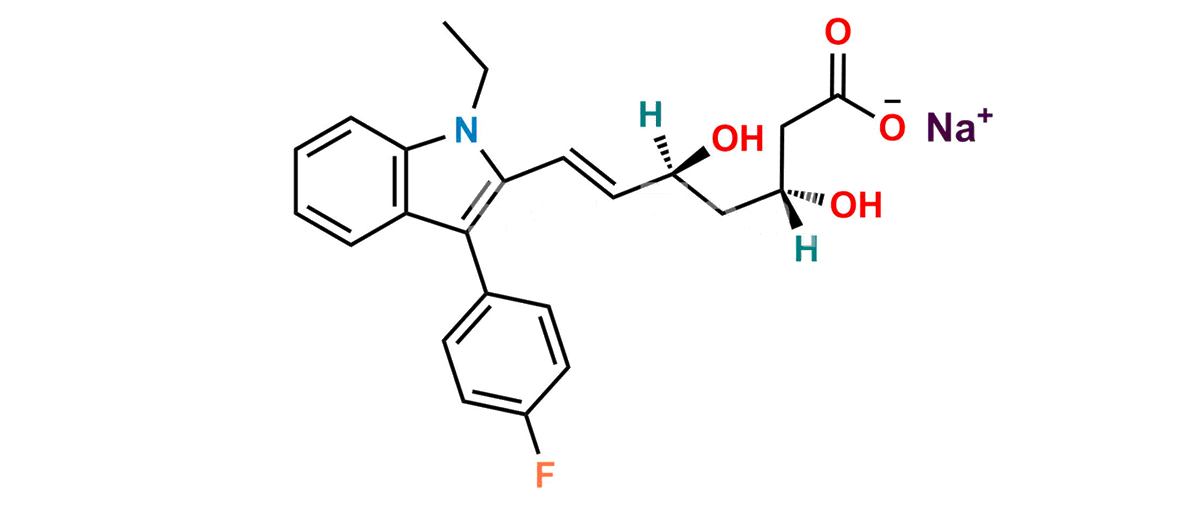
Fluvastatin EP Impurity C Sodium salt
Product Description
CAT No.
ALN-F025004
CAS No.
NA
Mol. F.
C23H23FNO4 : Na
Mol. Wt.
396.4 : 23.0
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
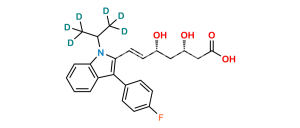
Chemical Name : Sodium (3R,5S,E)-7-(1-Ethyl-3-(4-fluorophenyl)-1H-indol-2-yl)-3,5-dihydroxyhept-6-enoate (as per EP); sodium (3R,5S,E)-7-[1-ethyl-3-(4-fluorophenyl)-1H-indol-2-yl]-3,5-dihydroxyhept-6-enoate (as per USP)
Smiles : O[C@](C[C@@]([H])(O)CC([O-])=O)([H])/C=C/C(N1CC)=C(C2=CC=C(F)C=C2)C3=C1C=CC=C3.[Na+]
Inchi : InChI=1S/C24H26FNO4.Na/c1-15(2)26-21-6-4-3-5-20(21)24(16-7-9-17(25)10-8-16)22(26)12-11-18(27)13-19(28)14-23(29)30;/h3-12,15,18-19,27-28H,13-14H2,1-2H3,(H,29,30);/b12-11+;/t18-,19-;/m0./s1
Synonym : Fluvastatin N-ethyl analog (USP)
Technical Data
Reference
Improvement of development and validation of an RP-HPLC method for the fluvastatin sodium using QbD approach and its application to forced degradation studies
By Balasaheb, Dhamdhere Rupali; Vijayalakshmi, A.nFrom International Journal of Research in Pharmaceutical Sciences (Madurai, India) (2020), 11(4), 6938-6948
Method development and validation of Fluvastatin by RP-HPLC
By Monika, Mallepally; Preethi, Ch. Sai; Aruna Rekha, D.; Pandey, Durgesh; Madhuri, G. – From World Journal of Pharmacy and Pharmaceutical Sciences (2020), 9(5), 1342-1351
Development and Validation of Stability-Indicating HPLC Methods for Quantitative Determination of Pravastatin, Fluvastatin, Atorvastatin, and Rosuvastatin in Pharmaceuticals
By Gomes, Fabio Pereira; Garcia, Pedro Lopez; Alves, Joao Marcel Porto; Singh, Anil Kumar; Kedor-Hackmann, Erika Rosa Maria; Santoro, Maria Ines Rocha Miritello – From Analytical Letters (2009), 42(12), 1784-1804
RFQ