No products in the cart.
Formoterol EP Impurity F

Product Description
CAT No.
ALN-F005007
CAS No.
1795129-59-7
Mol. F.
C37H46N4O6
Mol. Wt.
642.8
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
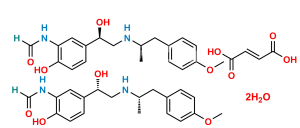
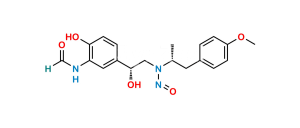
Chemical Name : N-[2-Hydroxy-5-[1-[[2-hydroxy-5-[1-hydroxy-2-[[2-(4-methoxyphenyl)-1-methylethyl]amino]ethyl]phenyl]amino]-2-[[2-(4-methoxyphenyl)-1-methylethyl]amino]ethyl]phenyl]formamide (as per EP);
N-[2-Hydroxy-5-(1-{[2-hydroxy-5-(1-hydroxy-2-{[1-(4-methoxyphenyl)propan-2-yl]amino}ethyl)phenyl]amino}-2-{[1-(4-methoxyphenyl)propan-2-yl]amino}ethyl)phenyl]formamide (as per USP)
Smiles : O=CNC1=CC(C(NC2=CC(C(O)CNC(C)CC3=CC=C(OC)C=C3)=CC=C2O)CNC(C)CC4=CC=C(OC)C=C4)=CC=C1O
Inchi : InChI=1/C20H26N2O4/c1-13-8-15(4-7-20(13)26-3)9-14(2)21-11-19(25)16-5-6-18(24)17(10-16)22-12-23/h4-8,10,12,14,19,21,24-25H,9,11H2,1-3H3,(H,22,23)
Synonym : Formoterol
dimer (USP)
Technical Data
Reference
A novel RP-HPLC Method for the Simultaneous Estimation of Aclidinium Bromide and Formoterol Fumarate in Bulk and Pharmaceutical Dosage Forms with Stability Studies
By Polisetty, Sai Suharshini; Guruva, Reddy M.nFrom International Journal of Pharmacy and Pharmaceutical Research (2020), 19(3), 530-544
Analytical method development and validation of formoterol and glycopyrrolatein pure and dosage forms by using RP-HPLC
By Mallikarjuna, R.; Reddy, L. Ramachandra; Dhachinamoorthi, D. – From European Journal of Biomedical and Pharmaceutical Sciences (2019), 6(10), 298-307
Simultaneous RP-HPLC method for determination of impurities in formoterol fumarate and aclidinium bromide in pharmaceutical dosage forms
By Gowda, Ravi; Sathe, Padmakar A. – From International Journal of Chemical and Pharmaceutical Analysis (2016), 3(3), 1-6
RFQ