No products in the cart.
Furosemide EP Impurity F
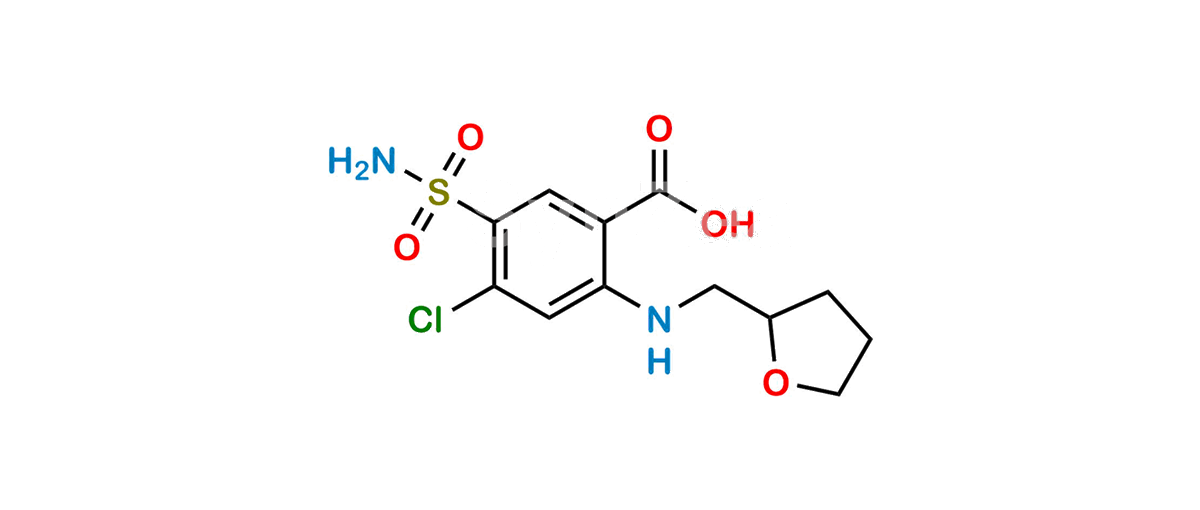
Product Description
CAT No.
ALN-F032007
CAS No.
4793-38-8
Mol. F.
C12H15ClN2O5S
Mol. Wt.
334.8
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
Chemical Name : 4-Chloro-5-sulfamoyl-2-[[[(2RS)-oxolan-2-yl]methyl]amino]benzoic acid
Smiles : O=C(O)C1=CC(S(=O)(N)=O)=C(Cl)C=C1NCC2OCCC2
Inchi : InChI=1S/C17H23N3O2.HI/c1-20(2,3)7-6-13-10-18-16-5-4-12(9-15(13)16)8-14-11-22-17(21)19-14;/h4-5,9-10,14,18H,6-8,11H2,1-3H3;1H/t14-;/m0./s1
Synonym : Tetrahydro Furosemide
Technical Data
Reference
A New Way of Stabilization of Furosemide upon Cryogenic Grinding by Using Acylated Saccharides Matrices. The Role of Hydrogen Bonds in Decomposition Mechanism
E. Kaminska,*,u2020 K. Adrjanowicz,u2021,u00a7 K. Kaminski,u00a7,u2225 P. Wlodarczyk,u22a5 L. Hawelek,u00a7,u22a5 K. Kolodziejczyk,u00a7 M. Tarnacka,u00a7 D. Zakowiecki,# I. Kaczmarczyk-Sedlak,u2020 J. Pilch,u2207 and M. PaluchnMol. Pharmaceutics 2013, 10, 5, 1824u20131835
Effect of Cryogrinding on Chemical Stability of the Sparingly Water-Soluble Drug Furosemide
,† K. Adrjanowicz,‡,§ K. Kaminski,§,∥ P. Wlodarczyk,⊥ L. Hawelek,§,⊥ K. Kolodziejczyk,§ M. Tarnacka,§ D. Zakowiecki,# I. Kaczmarczyk-Sedlak,† J. Pilch,∇ and M. Paluch – Mol. Pharmaceutics 2013, 10, 5, 1824–1835
Implementation of design of experiments for optimization of forced degradation conditions and development of a stability-indicating method for furosemide
Karolina Adrjanowicz, Kamil Kaminski, Katarzyna Grzybowska, Lukasz Hawelek, Marian Paluch, Irena Gruszka, Daniel Zakowiecki, Wieslaw Sawicki, Przemyslaw Lepek, Wojciech Kamysz & Lukasz Guzik – Pharmaceutical Research volume 28, pages3220–3236(2011)
RFQ






