No products in the cart.
Gatifloxacin Impurity 2

Product Description
CAT No.
ALN-G004014
CAS No.
855997-06-7
Mol. F.
C16H18FN3O4
Mol. Wt.
335.3
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
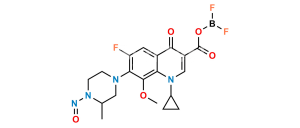
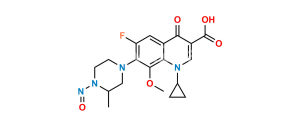
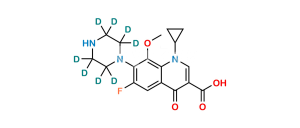
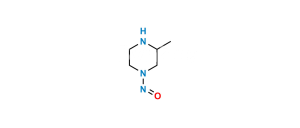
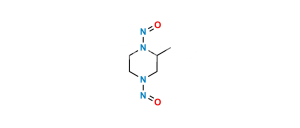
Chemical Name : 6-Fluoro-8-methoxy-7-(3-methylpiperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid
Smiles : COC(C(N(CCN1)CC1C)=C(F)C=C23)=C2NC=C(C(O)=O)C3=O
Inchi : InChI=1/C19H22FN3O5/c1-10-8-21(5-6-23(10)27)16-14(20)7-12-15(18(16)28-2)22(11-3-4-11)9-13(17(12)24)19(25)26/h7,9-11,27H,3-6,8H2,1-2H3,(H,25,26)
Technical Data
Reference
A High-Throughput Impurity-Free Process for Gatifloxacin
F. Javier Villasante,* Lourdes Gude, Sara P. Fernau00b4ndez, Olga Alonso, Elena Garcu0131u00b4a, and Antonio CosmenOrganic Process Research & Development 2008, 12, 900u2013903
Stability indicating RP-HPLC method for simultaneous determination of gatifloxacin and dexamethasone in binary combination
Lourdes Gude, Sara P. Ferna´ndez, Olga Alonso, Elena Garcı´a, and Antonio Cosme – Organic Process Research & Development 2008, 12, 900–903
A Validated, Specific, Stability-Indicating RP-LC Method for Analysis of Gatifloxacin in the Presence of Degradation Products and Process-Related Impurities
Syed Naeem Razzaq1 ,Muhammad Ashfaq2
RFQ