No products in the cart.
Gefitinib EP Impurity B

Product Description
CAT No.
ALN-G018003
CAS No.
1603814-04-5
Mol. F.
C22H24ClFN4O3
Mol. Wt.
446.9
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
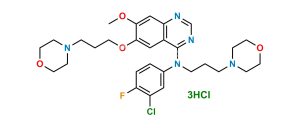
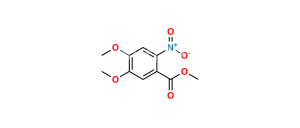
Chemical Name : N-(4-Chloro-3-fluorophenyl)-7-methoxy-6-(3-morpholinopropoxy)quinazolin-4-amine
Smiles : FC1=C(Cl)C=CC(NC2=NC=NC3=C2C=C(OCCCN4CCOCC4)C(OC)=C3)=C1
Inchi : InChI=1S/C16H21N3O4/c1-21-14-10-13-12(16(20)18-11-17-13)9-15(14)23-6-2-3-19-4-7-22-8-5-19/h9-11H,2-8H2,1H3,(H,17,18,20)
Technical Data
Reference
Identification, characterization and HPLC quantification of formulation-related impurities of honokiol, an antitumor natural drug candidate in clinical trials
Wenshuang Wu 1,2, # , Ming-Hai Tang2, # , Huan Tang 2 , Kai Chen 2,3 , Jie Fu 4 , Lun Wang 2,3 , Lin-Lin Xue 2 , Aihua Peng 2 , Haoyu Ye 2, 4* , Li-Juan ChennJournal of Pharmaceutical and Biomedical Analysis Volume 153, 10 May 2018, Pages 186-192
Separation and Estimation of Process-Related Impurities of Gefitinib by Reverse-Phase High-Performance Liquid Chromatography
, Li-Juan Chen – Journal of Pharmaceutical and Biomedical Analysis Volume 153, 10 May 2018, Pages 186-192
Karunakara A. Chandrashekara, Aparna Udupi, Chandrasekara G. Reddy – Journal of Chromatographic Science, Volume 52, Issue 8, September 2014, Pages 799–805
RFQ