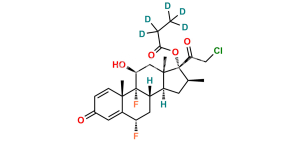
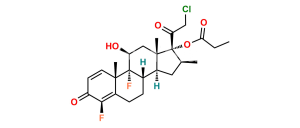
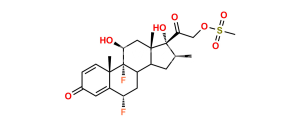
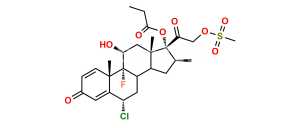
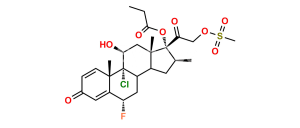
Halobetasol Impurity 6

Product Description
CAT No.
ALN-H011013
CAS No.
NA
Mol. F.
C27H36F2O6
Mol. Wt.
494.6
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
Chemical Name : (6S,9R,11S,16S,17R)-2′-Ethoxy-2′-ethyl-6,9-difluoro-11-hydroxy-10,13,16-trimethyl-7,8,9,10,11,12,13,14,15,16-decahydrospiro[cyclopenta[a]phenanthrene-17,4′-[1,3]dioxane]-3,5′(6H)-dione
Smiles : F[C@]12C(C(C[C@H](C)[C@@]34OC(CC)(OCC)OCC4=O)C3(C)C[C@@H]2O)C[C@H](F)C5=CC(C=CC51C)=O
Inchi : InChI=1S/C9H5Cl2N/c10-6-1-2-7-8(11)3-4-12-9(7)5-6/h1-5H
Technical Data
Reference
Quantification of halobetasol propionate and its impurities present in topical dosage forms by stability-indicating LC method
By Nalwade, Santaji; Reddy, Vangala Ranga; Kulkarni, Dipak; Todamal, SandipnFrom Journal of Chromatographic Science (2015), 53(1), 127-134
Impurity profiling and a stability-indicating UPLC method development and validation for the estimation of related impurities of halobetasol propionate in halobetasol propionate 0.05% (w/w) cream
By Prakash, Lakkireddy; Malipeddi, H.; Subbaiah, Venkata B.; Lakka, Narasimha S. – From Journal of Chromatographic Science (2015), 53(1), 112-121
Spectrophotometric determination of clobetasol propionate, halobetasol propionate, quinagolide hydrochloride, through charge transfer complexation
By Mostafa Azza A; Bebawy Lories I; Refaat Heba H – From Journal of pharmaceutical and biomedical analysis (2002), 27(6), 889-99
RFQ