No products in the cart.
7-Demethyl Ivabradine (HCl salt)

Product Description
CAT No.
ALN-I014045
CAS No.
NA
Mol. F.
C26H34N2O5 : HCl
Mol. Wt.
454.6 : 36.5
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
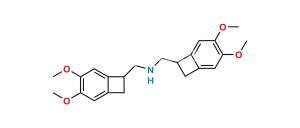
Chemical Name : (S)-3-(3-(((3,4-Dimethoxybicyclo[4.2.0]octa-1(6),2,4-trien-7-yl)methyl)(methyl)amino)propyl)-7-hydroxy-8-methoxy-1,3,4,5-tetrahydro-2H-benzo[d]azepin-2-one hydrochloride
Smiles : COC1=C(O)C=C(CCN(CCCN(C[C@@H]2C3=C(C=C(OC)C(OC)=C3)C2)C)C(C4)=O)C4=C1.Cl
Inchi : InChI=1S/C27H36N2O6.ClH/c1-28(16-19-11-18-13-23(33-3)24(34-4)14-20(18)19)8-6-9-29-10-7-17-12-22(32-2)25(35-5)15-21(17)26(30)27(29)31;/h12-15,19,26,30H,6-11,16H2,1-5H3;1H/t19-,26?;/m1./s1
Technical Data
Reference
Forced Degradation Studies of Ivabradine and In Silico Toxicology Predictions for Its New Designated Impurities
Piotr Pikul,1 Marzena Jamru00f3giewicz,1,* Joanna Nowakowska,1 Weronika Hewelt-Belka,2,3 and Krzesimir CiuranFront Pharmacol. 2016; 7: 117.
Simultaneous determination of chiral and achiral impurities of ivabradine on a cellulose tris(3-chloro-4-methylphenylcarbamate) chiral column using polar organic mode
Joanna Nowakowska,1 Weronika Hewelt-Belka,2,3 and Krzesimir Ciura – Front Pharmacol. 2016; 7: 117.
Characterization of degradation products of Ivabradine by LC-HR-MS/MS: a typical case of exhibition of different degradation behaviour in HCl and H2SO4 acid hydrolysis
ElekFerenczabBélaKovácsabFranciscBodaaMohammadhassanForoughbakhshfasaeicÉva KatalinKelemenbGerg?TóthcZoltán-IstvánSzabó – Journal of Pharmaceutical and Biomedical Analysis Volume 177, 5 January 2020, 112851
RFQ