No products in the cart.
Ledipasvir Acetyl Spiro Impurity

Product Description
CAT No.
ALN-L010037
CAS No.
2040487-36-1
Mol. F.
C44H45F2N7O4
Mol. Wt.
773.9
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
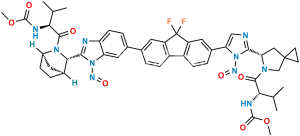
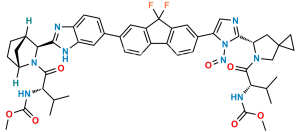
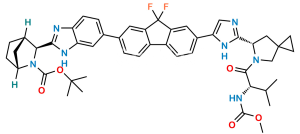
Chemical Name : Methyl ((S)-1-((1R,3S,4S)-3-(6-(7-(2-((S)-5-acetyl-5-azaspiro[2.4]heptan-6-yl)-1H-imidazol-5-yl)-9,9-difluoro-9H-fluoren-2-yl)-1H-benzo[d]imidazol-2-yl)-2-azabicyclo[2.2.1]heptan-2-yl)-3-methyl-1-oxobutan-2-yl)carbamate
Smiles : O=C(OC)N[C@@H](C(C)C)C(N1[C@](C2)([H])CC[C@]2([H])[C@H]1C3=NC4=CC=C(C5=CC(C(F)(F)C6=C7C=CC(C8=CN=C([C@H](C9)N(C(C)=O)CC%109CC%10)N8)=C6)=C7C=C5)C=C4N3)=O
Technical Data
Reference
Insights into the Dissolution Behavior of Ledipasvir-Copovidone Amorphous Solid Dispersions: Role of Drug Loading and Intermolecular Interactions
Chailu Que, Xiaochun Lou, Dmitry Y. Zemlyanov, Huaping Mo, Anura S. Indulkar, Yi Gao, Geoff G.Z. Zhang, and Lynne S. TaylornMol. Pharmaceutics 2019, 16, 12, 5054u20135067
A green stability-indicating RP-HPLC-UV method using factorial design for determination of ribavirin, sofosbuvir and ledipasvir: Application to average content, acid degradation kinetics and in vitro drug interactions study
Hanan I.EL-ShorbagyacFawziElsebaeiaSherin F.HammadbAmina M.El-Brashy – Microchemical Journal Volume 158, November 2020, 105251
Simultaneous chromatographic analysis of Sofosbuvir/Ledipasvir in their combined dosage form: an application to green analytical chemistry
Ahmed Hemdan & Maya S. Eissa – Journal of Analytical Science and Technology volume 10, Article number: 39 (2019)
RFQ