No products in the cart.
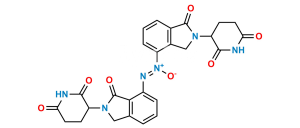
Lenalidomide Impurity 44

Product Description
CAT No.
ALN-L031064
CAS No.
NA
Mol. F.
C26H22N6O6
Mol. Wt.
514.5
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
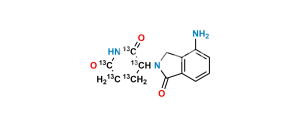
Chemical Name : (E)-3-(7-((2-(2,6-Dioxopiperidin-3-yl)-1-oxoisoindolin-4-yl)diazenyl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione
Smiles : O=C(C(N(CC1=C2C(/N=N/C3=CC=CC4=C3CN(C(CC5)C(NC5=O)=O)C4=O)=CC=C1)C2=O)CC6)NC6=O
Technical Data
Reference
Determination of possible potential genotoxic impurities in lenalidomide drug substance by simple RP-HPLC method
By Gaddam, Kishore; Kanne, Shanker; Gundala, Trivikram Reddy; Mamilla, Yogeshwar Reddy; Chinna, Gangi Reddy NallagondunFrom Asian Journal of Chemistry (2020), 32(12), 2965-2970
Development and validation of stability-indicating RP-HPLC method for the estimation of lenalidomide and its impurities in oral solid dosage form
By Prasad, Somana Siva; Mohan, G. V. Krishna; Babu, A. Naga – From Oriental Journal of Chemistry (2019), 35(1), 140-149
Simultaneous quantification of lenalidomide, ibrutinib and its active metabolite PCI-45227 in rat plasma by LC-MS/MS: Application to a pharmacokinetic study
By Veeraraghavan, Sridhar; Viswanadha, Srikant; Thappali, Satheeshmanikandan; Govindarajulu, Babu; Vakkalanka, Swaroopkumar; Rangasamy, Manivannan – From Journal of Pharmaceutical and Biomedical Analysis (2015), 107, 151-158
RFQ