No products in the cart.
Levonorgestrel EP Impurity A

Product Description
CAT No.
ALN-L021002
CAS No.
1260525-53-8
Mol. F.
C21H26O2
Mol. Wt.
310.4
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
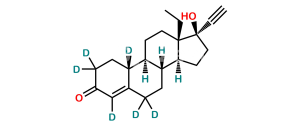
Chemical Name : 13-Ethyl-17-hydroxy-18,19-dinor-17α-pregna-4,8(14)-dien-20-yn-3-one (as per EP)
Smiles : O=C1CC[C@@]2([H])C(CCC3=C(CC[C@@]4(O)C#C)[C@]4(CC)CC[C@@]32[H])=C1
Inchi : InChI=1S/C21H28O2/c1-3-20-11-9-17-16-8-6-15(22)13-14(16)5-7-18(17)19(20)10-12-21(20,23)4-2/h2,13,16-19,23H,3,5-12H2,1H3/t16-,17+,18+,19-,20-,21-/m0/s1
Synonym : Norgestrel 8(14)-Dehydro Impurity
Technical Data
Reference
Comparative study on the application of capillary liquid chromatography and capillary electrochromatography for investigation of enantiomeric purity of the contraceptive drug levonorgestrel
Bezhan Chankvetadze a,b,, Irma Kartozia a , Chiyo Yamamoto c , Yoshio Okamoto c , Gottfried BlaschkenJournal of Pharmaceutical and Biomedical Analysis 30 (2003) 1897/1906
Analytical method development and method validation for determination assay and content uniformity of levonorgestrel by reversed-phase highperformance liquid chromatography
vikas kumar pal
, yogendra pal – Asian J Pharm Clin Res, Vol 13, Issue 4, 2020, 101-107
RFQ