No products in the cart.
Levonorgestrel EP Impurity B

Product Description
CAT No.
ALN-L021003
CAS No.
19914-67-1
Mol. F.
C21H28O2
Mol. Wt.
312.5
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
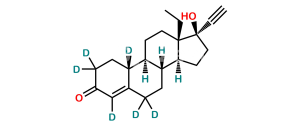
Chemical Name : 13-Ethyl-17-hydroxy-18,19-dinor-17α-pregn-5(10)-en-20-yn-3-one (as per EP)
Smiles : O=C1CCC2=C(CC[C@]3([H])[C@]2([H])CC[C@@]4(CC)[C@@]3([H])CC[C@@]4(O)C#C)C1
Inchi : InChI=1S/C21H26O2/c1-3-20-11-9-17-16-8-6-15(22)13-14(16)5-7-18(17)19(20)10-12-21(20,23)4-2/h2,13,16-17,23H,3,5-12H2,1H3/t16-,17+,20-,21-/m0/s1
Technical Data
Reference
Comparative study on the application of capillary liquid chromatography and capillary electrochromatography for investigation of enantiomeric purity of the contraceptive drug levonorgestrel
Bezhan Chankvetadze a,b,, Irma Kartozia a , Chiyo Yamamoto c , Yoshio Okamoto c , Gottfried BlaschkenJournal of Pharmaceutical and Biomedical Analysis 30 (2003) 1897/1906
Analytical method development and method validation for determination assay and content uniformity of levonorgestrel by reversed-phase highperformance liquid chromatography
vikas kumar pal
, yogendra pal – Asian J Pharm Clin Res, Vol 13, Issue 4, 2020, 101-107
RFQ