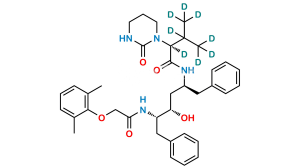
Lopinavir EP Impurity G

Product Description
CAT No.
ALN-L008008
CAS No.
NA
Mol. F.
C30H36N2O4
Mol. Wt.
488.6
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
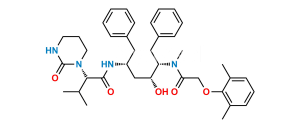
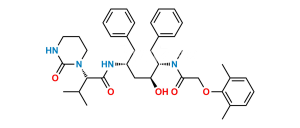
Chemical Name : N-[(1S,2S,4S)-(4-Acetylamino)-1-benzyl-2-hydroxy-5-phenylpentyl]-2-(2,6-dimethylphenoxy)acetamide (as per EP);
N-[(2S,3S,5S)-5-Acetamido-3-hydroxy-1,6-diphenylhexan-2-yl]-2-(2,6-dimethylphenoxy)acetamide (as per USP)
Smiles : O=C(N[C@@H](CC1=CC=CC=C1)[C@@H](O)C[C@@H](NC(C)=O)CC2=CC=CC=C2)COC3=C(C)C=CC=C3C
Inchi : InChI=1S/C29H34N2O4/c1-21-10-9-11-22(2)29(21)35-19-28(34)31-26(17-24-14-7-4-8-15-24)27(33)18-25(30-20-32)16-23-12-5-3-6-13-23/h3-15,20,25-27,33H,16-19H2,1-2H3,(H,30,32)(H,31,34)/t25-,26-,27-/m0/s1
Synonym : Lopinavir N-Acetylphenoxyacetamide (USP)
Technical Data
Reference
Stability indicative and cost effective creation and validation of analytical method of Lopinavir and Rilpivirine by high performance liquid chromatography
By Srinivas, L.; Jain, NeelunFrom International Journal of Research in Pharmaceutical Sciences (Madurai, India) (2021), 12(1), 786-792
Analytical methods development & validation for simultaneous estimation of lopinavir & ritonavir in pharmaceutical formulation by simultaneous equation method using UV spectrophotometry
By Jadhav, Sarika R.; Alhat, Hemant P.; Joshi, Suhas – From International Research Journal of Pharmacy (2018), 9(8), 57-62
Development and validation of forced degradation studies of lopinavir using RP-HPLC and characterization of degradants by LC-MS/MS
By Bhavyasri, Khagga; Balaram, V. Murali; Nageswarao, R.; Rambabu, D.; Ajitha, M. – From International Journal of Chemical Sciences (2015), 13(1), 551-562
RFQ