No products in the cart.
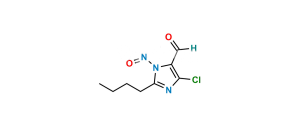
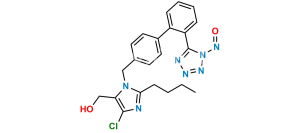
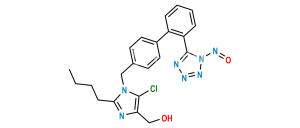
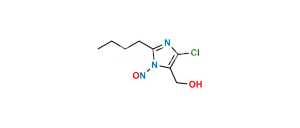
Losartan EP Impurity I

Product Description
CAT No.
ALN-L028010
CAS No.
1006062-28-7
Mol. F.
C41H37ClN6O
Mol. Wt.
665.2
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
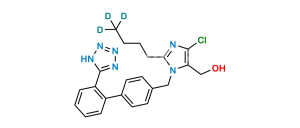
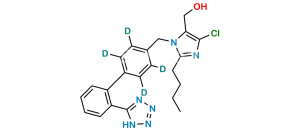
Chemical Name : 5-[4′-[[2-Butyl-4-chloro-5-[(triphenyl methoxy)methyl]-1H-imidazol-1-yl]methyl] [1,1′-biphenyl]-2-yl]-2H-tetrazole
Smiles : ClC1=C(COC(C2=CC=CC=C2)(C3=CC=CC=C3)C4=CC=CC=C4)N(CC5=CC=C(C6=CC=CC=C6C7=NNN=N7)C=C5)C(CCCC)=N1
Inchi : InChI=1S/C41H37ClN6O/c1-2-3-23-38-43-39(42)37(29-49)47(38)28-30-24-26-31(27-25-30)35-21-13-14-22-36(35)40-44-46-48(45-40)41(32-15-7-4-8-16-32,33-17-9-5-10-18-33)34-19-11-6-12-20-34/h4-22,24-27,49H,2-3,23,28-29H2,1H3
Synonym : O-Trityl Losartan ; Losartan Trityl Ether
Technical Data
Reference
Development and validation of a stability-indicating HPLC method for the simultaneous determination of Losartan potassium, hydrochlorothiazide, and their degradation products
Deanne L. Hertzog a,*, Jennifer Finnegan McCafferty b , Xueguang Fang b , R. Jeffrey Tyrrell c , Robert A. ReednJournal of Pharmaceutical and Biomedical Analysis 30 (2002) 747u2013760
An Efficient, Commercially Viable, and Safe Process for Preparation of Losartan Potassium, an Angiotensin II Receptor Antagonist
, Jennifer Finnegan McCafferty b , Xueguang Fang b , R. Jeffrey Tyrrell c , Robert A. Reed – Journal of Pharmaceutical and Biomedical Analysis 30 (2002) 747–760
Suri Babu Madasu,†,‡ N. A. Vekariya,
RFQ