No products in the cart.
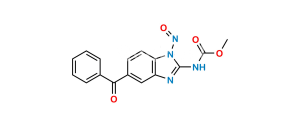
Mebendazole EP Impurity E

Product Description
CAT No.
ALN-M010006
CAS No.
31430-19-0
Mol. F.
C17H15N3O3
Mol. Wt.
309.3
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
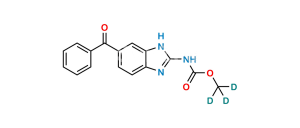
Chemical Name : N-(6-Benzoyl-1H-benzimidazol-2-yl)carbamic Acid Ethyl Ester; 5-Benzoyl-2-benzimidazolecarbamic Acid Ethyl Ester; (5-Benzoyl-1H-benzimidazol-2-yl)carbamic Acid Ethyl Ester;
Smiles : O=C(C1=CC(N=C(NC(OCC)=O)N2)=C2C=C1)C3=CC=CC=C3
Inchi : InChI=1S/C17H15N3O3/c1-20-14-9-8-12(15(21)11-6-4-3-5-7-11)10-13(14)18-16(20)19-17(22)23-2/h3-10H,1-2H3,(H,18,19,22)
Synonym : Mebendazole Ethyl Analog
Technical Data
Reference
High-performance liquid chromatographic separation and determination of the process related impurities of mebendazole, fenbendazole and albendazole in bulk drugs
Antony Raj Gomes *, V. NagarajunJournal of Pharmaceutical and Biomedical Analysis 26 (2001) 919u2013927
New RP-UPLC method development using QbD approach for determination of mebendazole, quinfamide, its impurities and antioxidants in mebendazole and quinfamide fixed dose combinations (FDC)
, V. Nagaraju – Journal of Pharmaceutical and Biomedical Analysis 26 (2001) 919–927
Rakesh Chandrakant Prabhu, Arthanareeswari Maruthapillai – Materials Today: Proceedings Volume 40, Supplement 1, 2021, Pages S120-S126
RFQ