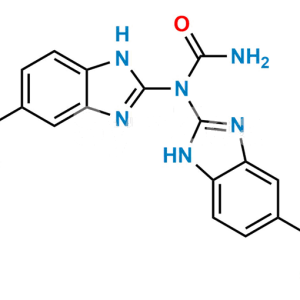
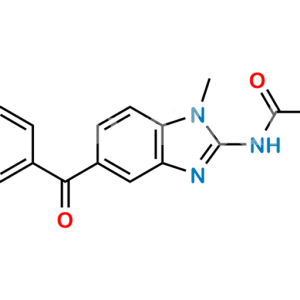
Mebendazole EP Impurity F

Product Description
CAT No.
ALN-M010007
CAS No.
31545-31-0
Mol. F.
C17H15N3O3
Mol. Wt.
309.3
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
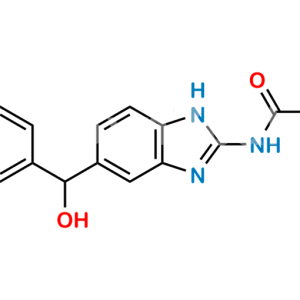
Chemical Name : [5-(4-Methylbenzoyl)-1H-benzimidazol-2-yl]carbamic Acid Methyl Ester; 5-p-Toluoyl-2-benzimidazolecarbamic Acid Methyl Ester;
Smiles : O=C(C1=CC(N=C(NC(OC)=O)N2)=C2C=C1)C3=CC=C(C)C=C3
Inchi : InChI=1S/C17H15N3O3/c1-2-23-17(22)20-16-18-13-9-8-12(10-14(13)19-16)15(21)11-6-4-3-5-7-11/h3-10H,2H2,1H3,(H2,18,19,20,22)
Synonym : 4-Methyl Mebendazole;
Technical Data
Reference
High-performance liquid chromatographic separation and determination of the process related impurities of mebendazole, fenbendazole and albendazole in bulk drugs
Antony Raj Gomes *, V. NagarajunJournal of Pharmaceutical and Biomedical Analysis 26 (2001) 919u2013927
New RP-UPLC method development using QbD approach for determination of mebendazole, quinfamide, its impurities and antioxidants in mebendazole and quinfamide fixed dose combinations (FDC)
, V. Nagaraju – Journal of Pharmaceutical and Biomedical Analysis 26 (2001) 919–927
Rakesh Chandrakant Prabhu, Arthanareeswari Maruthapillai – Materials Today: Proceedings Volume 40, Supplement 1, 2021, Pages S120-S126
RFQ