No products in the cart.
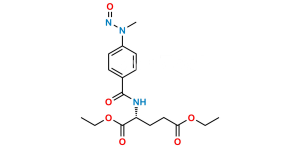
Methotrexate EP Impurity G

Product Description
CAT No.
ALN-M019008
CAS No.
2519848-42-9
Mol. F.
C28H29N9O6
Mol. Wt.
587.6
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
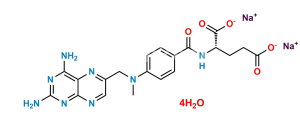
Chemical Name : (4-(4-(((2,4-diamino-4a,8a-dihydropteridin-6-yl)methyl)(methyl)amino)-N-methylbenzamido)benzoyl)-L-glutamic acid
Smiles : NC1=NC(N=CC(CN(C)C2=CC=C(C(N(C3=CC=C(C(N[C@@](CCC(O)=O)(C(O)=O)[H])=O)C=C3)C)=O)C=C2)=N4)=C4C(N)=N1
Inchi : InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m1/s1
Technical Data
Reference
Identification and Quantitation of Impurities in Methotrexate
dulal c. chatterji, altice g. frazier, and joseph f. gallellnJournal of Pharmaceutical Sciences Volume 67, Issue 5, May 1978, Pages 622-624
determination of methotrexate and its major metabolite, 7_hydroxymethotrexate, using capillary zone electrophoresis and laser-induced fluorescence detection
mark c. roach, philippe gozel and richard n. zare – Journal of Chromatography, 426 (1988) 129-140
Analytical methodologies for determination of methotrexate and its metabolites in pharmaceutical, biological and environmental samples
Forough Karami , Sara Ranjbar\xa0 , Younes Ghasemi , Manica Negahdaripour – Journal of Pharmaceutical Analysis 9 (2019) 373-391
RFQ