No products in the cart.
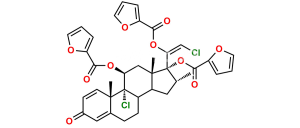
Mometasone EP Impurity Q

Product Description
CAT No.
ALN-M030013
CAS No.
83881-08-7
Mol. F.
C22H27ClO4
Mol. Wt.
390.9
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
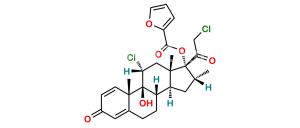
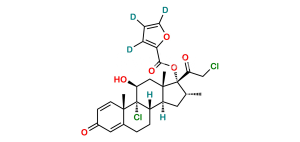
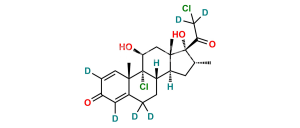
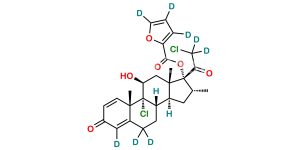
Chemical Name : 21-Chloro-9,11β-epoxy-17-hydroxy-16α-methyl-9β-pregna-1,4-diene-3,20-dione (as per EP)
Smiles : O[C@]1(C(CCl)=O)[C@H](C)C[C@@]2([H])[C@]3([H])CCC4=CC(C=C[C@]4(C)[C@]35[C@@H](O5)C[C@]12C)=O
Inchi : InChI=1S/C26H29ClO7/c1-14-10-19-18-7-5-15-11-16(29)6-8-17(15)25(18,27)21(30)12-24(19,2)26(14,22(31)13-28)34-23(32)20-4-3-9-33-20/h3-4,6,8-9,11,14,17-19,21,28,30H,5,7,10,12-13H2,1-2H3/t14-,17+,18+,19+,21+,24+,25+,26+/m1/s1
Technical Data
Reference
Simultaneous determination of Mometasone Furoate and Benzalkonium Chloride-A stability indicating method
By Kulkarni, Pankaj N.; Dodake-Supekar, Alaknanda M.; Gill, Charansingh H.nFrom Rasayan Journal of Chemistry (2020), 13(3), 1522-1530
Simultaneous estimation and validation of terbinafine hydrocloride and mometasone furoate in bulk and pharmaceutical dosage form by using RP-HPLC
By Khatik, Dipak P.; Lawre, R. B.; Mankar, S. D. – From World Journal of Pharmacy and Pharmaceutical Sciences (2019), 8(6), 1264-1274
Study of Intrinsic Stability of Mometasone Furoate in Presence of Salicylic Acid by HPTLC and Characterization, Cytotoxicity Testing of Major Degradation Product of Mometasone Furoate
By Vichare, Vijaya; Choudhari, Vishnu P.; Reddy, M. Venkata – From Current Pharmaceutical Analysis (2019), 15(6), 592-603
RFQ